Are you looking for an essay on ‘Electrophoresis’? Find paragraphs, long and short essays on ‘Electrophoresis’ especially written for school and college students.
Essay # 1. Introduction to Electrophoresis:
Electrophoresis is the migration or separation of charged particles or solutes in an electrical field. Charged particles will migrate to the electrode of the opposite charge, i.e., positive ions (cations) will migrate to the cathode, the negative electrode, while negative ions (anions) will migrate to the anode, the positive electrode. In gel electrophoresis, the molecules will travel at different speeds depending on their size. Smaller molecules will be able to move faster and will reach the far end of the gel, while larger molecules will be slowed down and remain near the beginning. This property can be utilized for the separation of proteins and DNA according to their size.
An amphoteric molecule has the ability to be negatively or positively charged depending upon the pH. A molecule with this amphoteric ability is referred as an ampholyte or Zwitter ion. Proteins and nucleic acids are amphoteric in nature; proteins with their ionizable amino and carboxyl groups are amphoteric. Thus by electrophoresis, usually, a lane with a standard marker is included and helps to determine relative sizes of samples. For example, the sizes of DNA and RNA markers are expressed in bases (b) or kilobases (kb), while the proteins markers are in Kilo Daltons.
Essay # 2. Types of Electrophoresis:
a. Sodium Dodecyl Sulfate-Polyacrvlamide Gel Electrophoresis (PAGE):
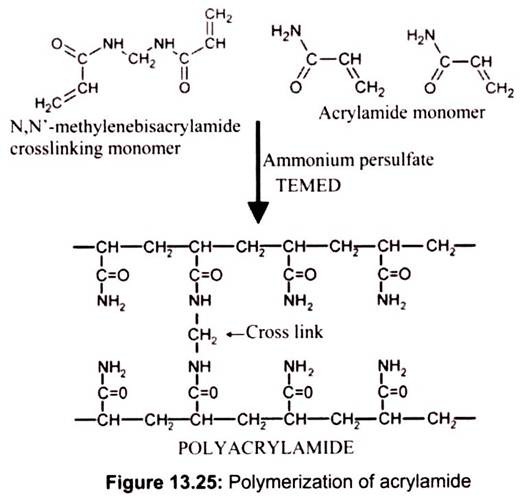
Polyacrylamide gel electrophoresis (PAGE) is probably the most common analytical technique used to separate and characterize proteins. In the process of polyacrylamide gel electrophoresis (PAGE), proteins are placed in an electric field and are forced to move through a porous matrix or gel (polyacrylamide) by the current. It involves the polymerization of solutions of acrylamide and bisacrylamide. The chemistry of the reaction consists of acrylamide forms linear polymers, while the bisacrylamide introduces cross links between polyacrylamide chains.
The ‘pore size’ is determined by the ratio of acrylamide to bisacrylamide, and by the concentration of acrylamide. A high ratio of bisacrylamide to acrylamide and a high acrylamide concentration cause low electrophoretic mobility. Polymerization of acrylamide and bisacrylamide monomers is induced by ammonium persulfate (APS), which spontaneously decomposes to form free radicals. TEMED, a free radical stabilizer, is generally included to promote polymerization. Riboflavin (or riboflavin-5′-phosphate) may also be used as a source of free radicals often in combination with TEMED and ammonium persulfate. In the presence of light and oxygen, riboflavin is converted in to its leuco form, which is active in initiating polymerization. This is referred as photochemical polymerization (Fig. 13.25).
To move through the gel, the proteins must move through the pores of the polyacrylamide. The rate at which proteins move through the polyacrylamide gel is dependent on their charge and their mass. The charge of a protein is dependent on its constituent amino acids and the pH of the buffer. Proteins that carry a high degree of charge will move at a faster rate than those with a lower overall charge. In addition, the mobility of small polypeptides through the gel will be faster than larger polypeptides because they are less restricted by the pores.
The overall rate of movement or mobility of a protein in PAGE is determined by its charge to mass ratio or charge density. Proteins with a high charge to mass ratio will have a higher mobility in PAGE than those with a low ratio. Mercaptoethanol reduces all disulfide bonds of cysteine residues to free sulfhydryl groups, and heating with SDS disrupts all intra- and intermolecular protein interactions. This treatment yields individual polypeptide chains which carry an excess negative charge induced by the binding of the detergent, and an identical charge- mass ratio.
Working:
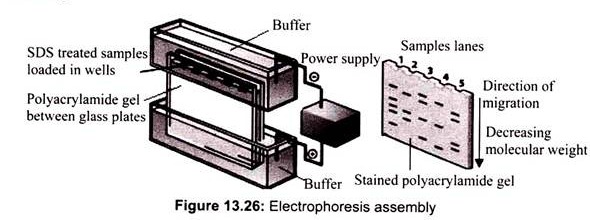
The process of SDS-PAGE is performed in a polyacrlyamide gel that is cast between two flat glass plates which are separated by spacers (1 mm thickness). The polyacrylamide gel consists of two separate components. The lower part of the gel is called the resolving gel. It is the portion of the gel that will act as molecular sieve to separate proteins. The upper part of the gel is called the stacking gel. The polyacrylamide content of the stacking gel is very low. Therefore, it has pores that are too large to separate proteins. The stacking gel contains several regularly spaced wells into which the samples will be loaded. In addition, the buffer in the stacking gel has particular electrical properties that will serve to concentrate the protein samples before they enter the resolving gel. This will maximize the separation or resolution of the gel (Fig. 13.26).
The electrophoretic apparatus contains two chambers: upper and lower buffer reservoirs. The glass plates containing the gel are clamped vertically into an apparatus. When the upper reservoir is filled with buffer, the buffer flows over the top edge of the shorter plate and contacts the upper surface of the gel. Buffer placed in the lower reservoir makes contact with the lower surface of the gel. Both reservoirs contain electrodes that are connected to a power supply. When the power is applied to the apparatus, the upper electrode acts as a cathode (negative electrode) and the lower electrode acts as an anode (positive electrode). The circuit between the two electrodes is completed by the flow of current through the slab gel.
The protein sample is applied to the upper part of the slab gel (stacking gel) in separate wells. The protein sample contains SDS, protein, buffer, and glycerol or sucrose. The glycerol or sucrose adds density to the sample making it easier to layer on the surface of the gel. The proteins move down toward the anode when the current is applied. The blue dye, bro- mophenol blue, is included in the sample. The dye is very small and highly charged. Therefore, it will move down through the gel at a rate faster than the proteins in the sample. The movement of the dye is used to monitor the progress of electrophoresis and is called the tracking dye. The electrophoresis is stopped when the blue dye reaches the bottom of the slab gel. After electrophoresis is finished, the gel is removed from between the glass plates and is incubated in a solution containing the dye, the Coomassie blue R-250. It will bind to the proteins within the gel and allows the visualization; the proteins will appear as blue bands.
Estimation of protein molecular weight by SDS-PAGE is a widely employed procedure. The relative mobility of a protein in an SDS-PAGE gel is related to its molecular weight. A standard curve is constructed with proteins of known molecular weight by plotting the logarithms of their molecular weights versus the relative mobilities of the proteins. The relative mobility of a protein of unknown molecular weight is then fitted to the curve to determine its molecular weight.
b. Native Gel Electrophoresis:
Native structure and conformation is very important for biological protein functions such as enzymatic activity and interaction of antibodies with their ligands, etc. To preserve native protein structure and conformation, proteins are analyzed by non-denaturing electrophoresis. The pH of the buffer system is an important parameter for optimal protein separation. Native protein electrophoresis is often carried out in Tris-glycine electrophoresis buffer. Protein samples used for the native electrophoresis should be devoid of strong denaturants such as SDS.
c. Agarose Gel Electrophoresis:
Agarose is a polysaccharide extracted from seaweed. Agarose gel electrophoresis is the easiest and common way of separating and analyzing DNA. The DNA separated by agarose gel electrophoresis is visualized by addition of ethidium bromide in the gel. This binds strongly to DNA by intercalating between the bases and is fluorescent; it absorbs UV light and transmits the energy as orange light.
Most agarose gels are made between 0.7% to 2%. A 0.7% gel will show good separation (resolution) of large DNA fragments (5-10 kb),while 2% gel will show good resolution for small fragments (0.2-1 kb). Typically, a band is easily visible if it contains about 20 ng of DNA. The loading buffer gives color and density to the sample to make it easy to load into the wells. Also, the dyes are negatively charged in neutral buffers and thus move in the same direction as the DNA during electrophoresis. This allows monitoring the progress of the gel. The most common dyes are bromophenol blue and xylene cyanol. Density is provided by glycerol or sucrose. Bromophenol blue migrates at a rate equivalent to 200-400 bp DNA. Xylene cyanol migrates at approximately 4kb equivalence.
Working:
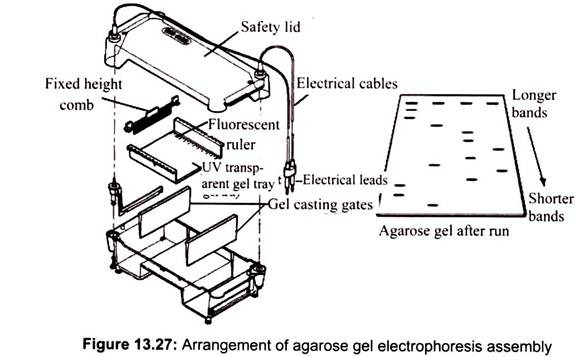
To cast the gel, agarose powder is mixed with electrophoresis buffer to the desired concentration and then heated in a microwave oven until completely melted. Most commonly, ethidium bromide is added to the gel (final concentration 0.5 ug/ml) at this point to facilitate visualization of DNA after electrophoresis. After cooling the solution to about 60°C, it is poured into a casting tray containing a sample comb and allowed to solidify at room temperature (Fig. 13.27).
Agarose gels can be run in many different types of electrophoresis buffers. Nucleic acid agarose gel electrophoresis is usually conducted with either Tris-Acetate-EDTA (TAE) buffer or Tris-Borate-EDTA (TBE) buffer. While TAE buffer provides faster electrophoretic migration of linear DNA and better resolution of super-coiled DNA, TBE buffers have a stronger buffering capacity for longer or higher voltage electrophoresis runs. After the complete run the separated bands can be viewed in transilluminator, a UV chamber. Agarose gels can be used for the separation of DNA fragments ranging from 50 base pairs to several megabases (millions of bases) by electrophoresis.
Agarose gel electrophoresis can be used for:
i. Separation of restriction enzyme digested DNA including genomic DNA, prior to Southern Blot transfer and RNA prior to Northern transfer.
ii. Analysis of PCR products to assess for target DNA amplification.
iii. Allow the estimation of the size of DNA molecules using a DNA marker or ladder which contains DNA fragments of various known sizes.
iv. Allow the rough estimation of DNA quantity and quality.
v. Other techniques rely on agarose gel electrophoresis for DNA separation including DNA fingerprinting.
d. Pulsed Field Electrophoresis:
It is a technique in which the direction of current flow in the electrophoresis chamber is periodically altered. This allows fractionation of pieces of DNA ranging from 50,000 to 5 millon bp, which is much larger than can be resolved on standard gels.
e. Alkaline Agarose Gels:
These are prepared with and electrophoresed in buffers containing sodium hydroxide. Such alkaline conditions are useful for analyzing single-stranded DNA.
f. Capillary Electrophoresis:
Capillary electrophoresis (CE) is a fairly recent separation technique, developed in the 1980s. It offers vast improvements over conventional electrophoresis methods. It is complementary to liquid chromatography and plays an important role among the different analytical techniques currently available. Capillary electrophoresis has numerous applications and provides the advantage of high resolution, speed, and ease of use, automation and low cost. CE can be applied to a wide range of compounds, ranging from small ions to macromolecules. Numerous CE methods have been developed for pharmaceutical, biological, phyto- chemical and environmental applications.
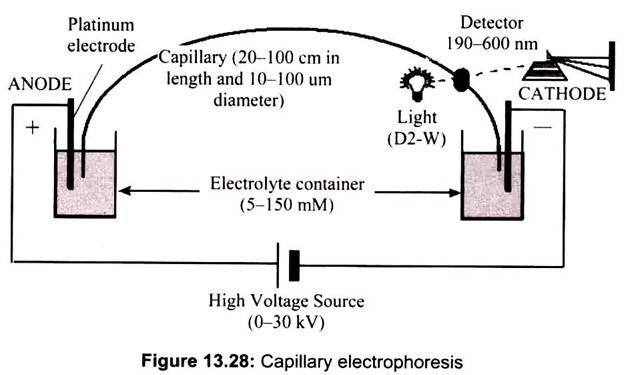
Capillary electrophoresis applies to molecules that are either positively or negatively charged and which migrate in an electrical field with different velocities. The time- dependent separations of the different constituents in a mixture depend on two principal factors called electrophoretic mobility and electro-osmotic flow. Indeed, a charged molecule in an electrical field is subjected to a force that is proportional to its effective charge (q) and the electrical field which applied (E). Neutral species are not separated unless there is some association with the ions of the electrolyte itself, leading to different drag forces. Different mobilities are designated as (+) or (-) depending on the charge that is carried. For species that have no net charge, mobility is nil (Fig. 13.28).
Electroosmotic flow (or electroosmolarity) finds its origins in the inner wall of the capillary which is lined with silanol groups. Silanols are compounds containing silicon atoms to which hydroxy substituents bond directly. These groups become ionized at pH 2 and above and thus create a negatively charged inner lining. To maintain electrical neutrality the cations from the buffer solution cover this lining, thus creating a second layer.
When an electrical field is created, the cations from this double layer start migrating towards the cathode. Their movement drags the buffer solution and creates a flow called the electroosmotic flow. This flow can be controlled by changing the electrical tension, the pH, the type of capillary used and by including specific additives. Because of electroosmotic flow, neutral molecules and anions may move in the direction of the detector which is usually located on the cathode end of the capillary.
Essay # 3. Instrumentation of Electrophoresis:
The instrumentation needed to perform capillary electrophoresis is very simple. Schematically, CE is composed of a silica capillary with both extremities resting in tanks filled with a buffer solution. Capillaries are usually 30-100 cm long and have an internal diameter of 50 or 75 μm. An electrical field of up to 30 kV may be applied through electrodes immersed in the electrolyte solution in the two tanks. The electrolyte solution is generally made up of an aqueous buffer with precise pH and ionic strength. It may also contain specific additives (e.g. cyclodextrins, surfactants, organic modifiers) that enhance the separation of molecules.
In order to avoid Joule heating, the current and the voltage are rigorously controlled and electrophoresis takes place in a thermostatic chamber.
The different compounds separated by electrophoresis are generally detected during the run by a UV-visible absorbance detector or a fluorometer positioned at the cathode end of the capillary. Now it is possible to couple CE to a mass spectrometer (MS) in real time. Besides adding sensitivity and selectivity, MS also provides structural information about the separated molecules.
This coupled technique can thus be used to analyze pharmaceutically active compounds and their metabolites in any biological fluid (e.g. plasma, urine, saliva). The coupling of the two techniques requires the addition of a specific solution to the effluent from the capillary. The mixture is then vaporized, ionized and finally introduced into the mass spectrometer.
There are two main methods to introduce the sample into the capillary- hydrodynamic injection (by pressure, aspiration or siphoning) and electrokinetic injection. The latter method is particularly useful in the detection of pharmaceutical product present in the ppb range. The injection volumes may be as low as a nanoliter and a few microliters of the sample are sufficient to carry out the entire analysis. Because of such small volumes, very expensive and even exotic additives can be used in developing new electrophoresis methods. In electrophoresis, strong electrical fields allow very rapid separations. Another advantage of capillary electrophoresis is its capacity to generate 400,000 to 1 million theoretical plates. As a diagnostic technique, electrophoresis relies on the difference in the mobilities of two distinct substances subjected to an electrical field.