Are you looking for an essay on ‘Centrifugation’? Find paragraphs, long and short essays on ‘Centrifugation’ especially written for school and college students.
Essay # 1. Introduction to Centrifugation:
Centrifugation is the method of separating immiscible liquids or solids from liquids by the application of centrifugal force. Centrifugation is one of the most important and widely applied research techniques in biochemistry, cellular and molecular biology, and in medicine. Current research and clinical applications rely on isolation of cells, subcellular organelles, and macromolecules.
The theoretical basis of this technique is the effect of gravity on particles (including macromolecules) in suspension. Two particles of different masses will settle in a tube at different rates in response to gravity. Centrifugal force is used to increase this settling rate in the centrifuge. Centrifuges achieve separation by means of the accelerated gravitational force achieved by a rapid rotation or simply a centrifuge uses centrifugal force to isolate suspended particles from their surrounding medium on either a batch or a continuous-flow basis.
An object traveling in a circle behaves as if it is experiencing an outward force. This force, known as the centrifugal force, depends on the mass of the object, the speed of rotation, and the distance from the center. The more massive the object, the greater the force; the greater the speed of the object, the greater the force (Centrifugal force Fc = mv2/r, where m = mass, v = speed, and r = radius).
The two common types of centrifugation are analytical and preparative; the distinction between the two is based on the purpose of centrifugation.
Analytical centrifugation involves measuring the physical properties of the sedimenting particles such as sedimentation coefficient or molecular weight. Optimal methods are used in analytical ultracentrifugation. Molecules are observed by optical system during centrifugation, to allow observation of macromolecules in solution as they move in gravitational field. The concentration of the solution at various points in the cell is determined by absorption of a light of the appropriate wavelength (Beer’s law is followed).
The other form of centrifugation is called preparative and the objective is to isolate specific particles which can be reused.
The four basic types of centrifuges that are used commonly in the laboratory are:
i. Clinical Centrifuges:
They are tabletop centrifuges that can run at a speed of up to 3000 rpm. These can pellet cells, but not organelles or biomolecules.
ii. Microfuges:
These can typically run at a speed of up to 14,000 rpm, sufficient speed to pellet nucleic acids and denatured proteins. Microfuges are specially adapted for small volumes of sample.
iv. High Speed Centrifuges:
They can run at speeds up to 25,000 rpm, sufficient to pellet cell nuclei and most biomolecules. These are often aided with cooling system.
v. Ultracentrifuges:
The ultracentrifuge is capable of reaching even greater velocities and requires a vacuum to reduce friction and heating of the rotor. It can run at speeds Up to 75,000 rpm, sufficient to allow fractionation of biomolecules, for example- plasmid DNA, chromosomal DNA, and RNA. Ultracentrifuges are usually refrigerated and are very expensive and have delicate pieces of machinery.
Essay # 2. Mechanical Components of Centrifuges:
(a) Rotors:
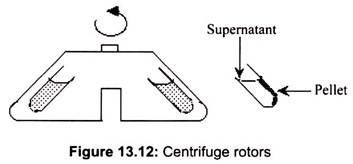
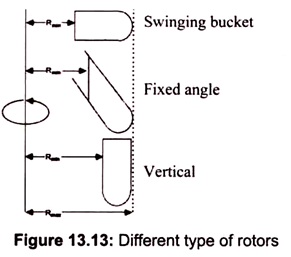
Rotors for a centrifuge are fixed angles, swinging buckets, continuous flow, or zonal. Fixed angles generally work faster; substances precipitate faster in a. given rotational environment. The most common is a rotor holding 8 centrifuge tubes at an angle of 34° from the vertical.
In a fixed angle rotor, the materials are forced against the side of the centrifuge tube, and then slide down the wall of the tube. This action is the primary reason for their apparent faster separation, but also leads to abrasion of the particles along the wall of the centrifuge tube (Fig. 13.12).
For swinging bucket rotors, the materials must travel down the entire length of the centrifuge tube and always through the media within the tube. Since the media is usually a viscous substance, the swinging bucket appears to have a lower relative centrifugal force, which takes longer to precipitate anything contained within. Most common clinical centrifuges have swinging buckets. Cell biologists employ zonal rotors for the large scale separation of particles on density gradients. Zonal rotors can contain up to 2 liters of solution (Fig. 13.13).
(b) Rotor Tubes:
The tubes used for biological work, are made of regular glass, Corex glass, or nitrocellulose. Regular glass centrifuge tubes can be used at speeds below 3,000 RPM that is in a standard clinical centrifuge. For work in the higher speed ranges, centrifuge tubes are made of plastic or nitrocellulose. Preparative centrifuge tubes are made of polypropylene (sometimes polyethylene) and can withstand speeds up to 20,000 RPM. These tubes should be carefully examined for stress fractures before use.
Essay # 3. Relative Centrifugal Force (RCF):
The centrifugal force is usually compared to the force of gravity and is reported as the relative centrifugal force (RCF) in g units.
The following formula relates the RCF to the rotor speed and diameter:
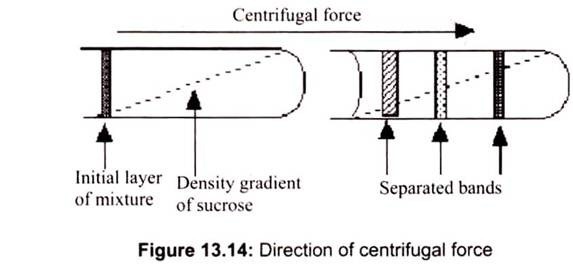
RCF = (1.119 x 10-5) (rpm) r
Where r is the radius of rotation (the radius of the rotor) in cm and rpm is the centrifuge speed in revolutions per minute. The number 1.119 x 10-5 is a conversion factor that allows to use “rpm” and “g” units (Fig. 13.14). When a suspension is rotated at a certain speed or revolutions per minute (RPM), centrifugal force causes the particles to move radially away from the axis of rotation. The force on the particles (compared to gravity) is called Relative Centrifugal Force (RCF).
Essay # 4. Types of Centrifugal Separations:
a. Differential Centrifugation:
In differential centrifugation separation is primarily based on the size of the particles. This type of separation is commonly used in simple pelleting and in obtaining partially-pure preparation of subcellular organelles and macromolecules. For the study of subcellular organelles, tissue or cells are first disrupted to release their internal contents. This crude disrupted cell mixture is referred to as a homogenate.
During centrifugation of a cell homogenate, larger particles sediment faster than smaller ones and this provides the basis for obtaining crude organelle fractions by differential centrifugation. Some of these sedimenting organelles can be obtained in partial purity and are typically contaminated with other particles. Repeated washing of the pellets by re-suspending in isotonic solvents and re-pelleting may result in removal of contaminants that are smaller in size.
b. Density Gradient Centrifugation:
Density gradient centrifugation is the preferred method to purify sub cellular organelles and macromolecules. Density gradients can be generated by placing layer after layer of gradient media such as sucrose in a tube with the heaviest layer at the bottom and the lightest at the top in either a discontinuous or continuous mode. The cell fraction to be separated is placed on top of the layer and centrifuged.
Density gradient separation can be classified into two categories:
i. Rate-zonal (size) separation and
ii. Isopycnic (density) separation.
i. Rate Zonal (Size) Separation:
Rate-zonal separation takes advantage of particle size and mass instead of particle density for sedimentation. Examples of common applications include separation of cellular organelles such as endosomes or separation of proteins, such as antibodies. For instance, antibody classes all have very similar densities, but different masses. Thus, separation based on mass will separate the different classes, whereas separation based on density will not be able to resolve these antibody classes.
In rate zonal centrifugation, the sample is applied in a thin zone at the top of the centrifuge tube on a density gradient. Under centrifugal force, the particles will begin sedimenting through the gradient in separate zones according to their size, shape, and density or the sedimentation coefficient(s). The run must be terminated before any of the separated particles reach the bottom of the tube. S is the sedimentation coefficient and is usually expressed in Svedbergs (S) units.
S is increases with mass of the particle because sedimenting force α M (1-νρ). S is also increased for more compact structures of equal particle mass (frictional coefficient is less)
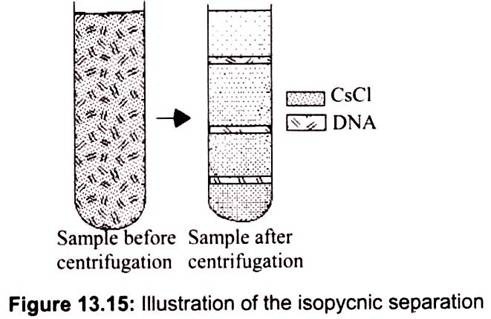
ii. Isopycnic Separation:
Molecules separated on equilibrium position and not by rates of sedimentation. Each molecule floats or sinks to position where density equals density of CsCl solution. In the isopycnic technique, the density gradient column encompasses the whole range of densities of the sample particles. The sample is uniformly mixed with the gradient material. Each of the particles will sediment only to the position in the centrifuge tube at which the gradient density is equal to its own density, and there it will remain.
The isopycnic technique, therefore, separates particles into zones solely on the basis of their buoyant density differences, independent of time. A common example for this method is separation of nucleic acids in a CsCl gradient. Isopynically banding DNA, for example, takes 36 to 48 hours in a self- generating cesium chloride gradient. It is important to note that the run time cannot be shortened by increasing the rotor speed; this only results in changing the position of the zones in the tube since the gradient material will redistribute further down the tube under greater centrifugal force (Fig. 13.15).