In this essay we will discuss about the various types of blotting techniques where molecules have been separated on a gel are transferred or blotted onto a type of paper called nitrocellulose.
1. Western Blotting:
Western blotting is an immunoblotting technique; rely on the specificity of binding between a molecule of interest and a probe to allow detection of the molecule of interest in a mixture of many other similar molecules. In Western blotting, the molecule of interest is a protein and the probe is typically an antibody raised against that particular protein.
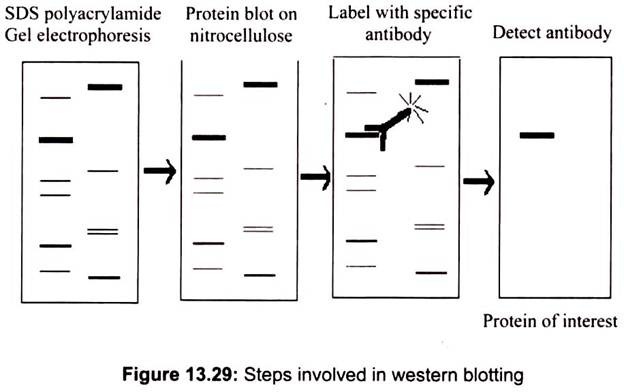
It is an analytical method wherein a protein sample is electrophoresed on SDS-PAGE and electro-transferred onto nitrocellulose membrane. The nitrocellulose is then soaked in blocking buffer (3% skimmed milk solution) to “block” the nonspecific binding of proteins. The nitrocellulose is then incubated with the specific antibody for the protein of interest. The nitrocellulose is then incubated with a second antibody, which is specific for the first antibody. The second antibody will typically have a covalently attached enzyme which, when provided with a chromogenic substrate, will cause a color reaction. Thus molecular weight and amount of desired protein can be characterized from complex mixture (Fig. 13.29).
The Western blot is primarily used in medical diagnostic applications like testing for mad-cow disease. The confirmatory HIV test also uses a Western blot. In certain cases, forensics uses Western blotting techniques to determine DNA data as well.
2. Southern Blotting:
Southern blotting was named after Edward M. Southern who developed this procedure at Edinburgh University in the 1970s. Southern blotting is designed to locate a particular sequence of DNA within a complex mixture. For example, Southern Blotting could be used to locate a particular gene within an entire genome. The amount of DNA needed for this technique is dependent on the size and specific activity of the probe. Short probes tend to be more specific. Under optimal conditions, it can detect up to 0.1 pg of the DNA.
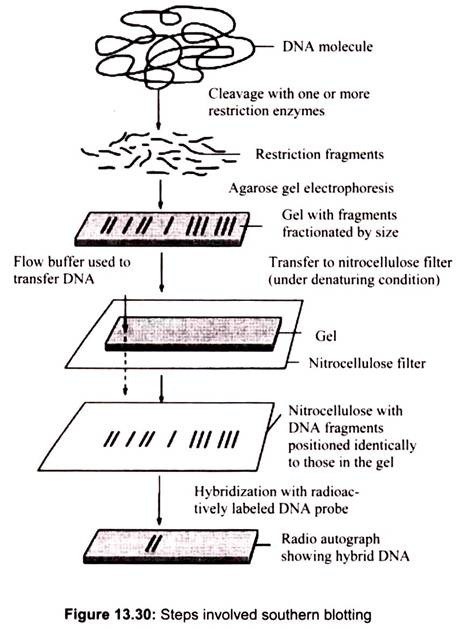
Interpretation of southern blot will help to assess whether a particular gene is present and how many copies are present in the genome of an organism and the degree of similarity between the chromosomal gene and the probe sequence. It also helps to find out whether recognition sites for particular restriction endonucleases are present in the gene. By performing the digestion with different endonucleases, or with combinations of endonucleases, it is possible to obtain a restriction map of the gene, i.e. an idea of the restriction enzyme sites in and around the gene- which will assist in attempts to clone the gene (Fig. 13.30).
3. Northern Blotting:
Northern blotting is a simple extension of Southern blotting. It is used to detect cellular RNA rather than DNA. After denaturation RNA will bind efficiently to nitrocellulose. This means that the RNA has to be unfolded into a linear strand before it will bind efficiently to nitrocellulose. Chemicals such as formaldehyde and methylmercuric hydroxide can be used to denature the RNA – breaking down hydrogen bonding structure in the molecule. Alkali is not used to denature the RNA – since RNA is degraded under alkaline conditions. Rests of the techniques are similar to that of southern blotting.
Northern blotting can tell us differential expression patterns of a particular gene in tissues or at which stages of development it gets expressed. The quantity of the mRNA present can be measured accurately by radioactive counting.