Are you looking for an essay on ‘Chromatography and Its Types’? Find paragraphs, long and short essays on ‘Chromatography and Its Types’ especially written for school and college students.
Essay # 1. Introduction to Chromatography:
Chromatography, literally “color writing”, was used primarily for the separation of plant pigments such as chlorophyll. New forms of chromatography developed in the 1930s and 1940s made the technique useful for a wide range of separation processes and chemical analysis tasks, especially in biochemistry. The first true chromatography is usually attributed to Russian botanist Mikhail Semyonovich Tsvet, who used columns of calcium carbonate for separating plant pigments in the first decade of the 20th century during his research on chlorophyll. Chromatography may be preparative or analytical. Preparative chromatography seeks to separate the components of a mixture for further use. Analytical chromatography normally operates with smaller amounts of material and seeks to measure the relative proportions of analytes in a mixture.
All forms of chromatography work on the same principle. They all have a stationary phase (a solid, or a liquid supported on a solid) and a mobile phase (a liquid or a gas). The mobile phase flows through the stationary phase and carries the components of the mixture with it. Different components travel at different rates. It involves passing a mixture dissolved in a “mobile phase” through a stationary phase, which separates the analyte to be measured from other molecules in the mixture and allows it to be isolated.
The molecules in the test preparation will have different interactions with the stationary support leading to separation of similar molecules. Test molecules which display tighter interactions with the support will tend to move more slowly through the support than those molecules with weaker interactions. In this way, different types of molecules can be separated from each other as they move over the support material.
Essay # 2. Types of Chromatography:
The main types of chromatography that are in routine use are:
A. Paper Chromatography:
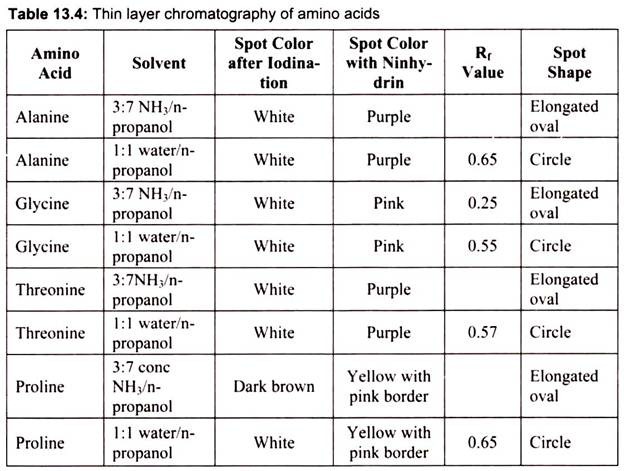
In paper chromatography, the stationary phase is a very uniform absorbent paper. The mobile phase is a suitable liquid solvent or mixture of solvents. Different compounds in the sample mixture travel different distances according to how strongly they interact with the paper. This paper is made of cellulose, a polar molecule, and the compounds within the mixture travel farther if they are non-polar. More polar substances bond with the cellulose paper more quickly, and therefore do not travel as far. This process allows the calculation of an Rf value and can be compared to standard compounds to aid in the identification of an unknown substance. It is generally used for the separation of amino acids, in which ninhydrin is used as spray reagent to visualize the spots.
B. Thin Layer Chromatography:
The stationary phase is a powdered adsorbent which consists of a thin layer of silica gel (SiO2) or alumina (AI2O3) coated on a glass or plastic sheet. The mixture to be analyzed is loaded near the bottom of the plate. The plate is placed in a reservoir of solvent so that only the bottom of the plate is submerged. This solvent is the mobile phase; it gradually moves up the plate via capillary action, and it carries the deposited substances along with it at different rates. The desired result is that each component of the deposited mixture is moved a different distance up the plate by the solvent. It is often used to judge the purity of a compound. The components appear as a series of spots at different locations up the plate.
Components can be identified from their so-called Rf values. The Rf value for a substance is the ratio of the distance that the substance travels to the distance that the solvent travels up the plate. In case of spots which are colorless can be viewed with the help of some coloring agents which specifically give color to the components in contrast background. For example, iodination will help to view the amino acids in a brown background.
C. High-Performance Thin Layer Chromatography (HPTLC):
High-Performance Thin Layer Chromatography is an analytical technique based on thin layer chromatography, but with enhancements intended to increase the resolution of the compounds to be separated and to allow quantitative analysis of the compounds. Some of the enhancements include, more accurate sample loading, use of a densitometer and computer analysis to determine the size, intensity and position (retardation factor) of the separated compounds and use of higher quality TLC plates with finer particle sizes in the stationary phase allowing better resolution.
D. Column Chromatography:
The stationary phase is a powdered adsorbent which is placed in a vertical glass column. The mixture to be analyzed is loaded on top of this column. The mobile phase is a solvent poured on top of the loaded column. The solvent flows down the column, causing the components of the mixture to distribute between the powdered adsorbent and the solvent, thus separating the components of the mixture so that as the solvent flows out of the bottom of the column, some components elute with early collections and other components elute with late fractions. The solvent is usually changed stepwise, and fractions are collected according to the separation required with the eluted solvent usually monitored by TLC.
E. High-Performance Liquid Chromatography (HPLC):
It is a form of column chromatography used frequently in biochemistry and analytical chemistry to separate, identify, and quantify compounds. HPLC utilizes a column that holds chromatographic packing material (stationary phase), a pump that moves the mobile phase(s) through the column, and a detector that shows the retention times of the molecules. Retention time varies depending on the interactions between the stationary phase, molecules being analyzed, and the solvent(s) used.
F. Reversed Phase Chromatography (RP-HPLC or RPC):
It is an adsorptive process by experimental design, which relies on a partitioning mechanism to affect separation. Reversed Phase Chromatography, results from the adsorption of hydrophobic molecules onto a hydrophobic solid support in a polar mobile phase. Decreasing the mobile phase polarity by using organic solvents reduces the hydrophobic interaction between the solute and the solid support resulting in de-sorption. The more hydrophobic the molecule the more avidly it will adsorb onto the solid support. This requires a higher concentration of organic solvent to promote de-sorption.
The binding of the analyte to the stationary phase is proportional to the contact surface area around the non-polar segment of the analyte molecule upon association with the ligand in the aqueous eluent. Structural properties of the analyte molecule play an important role in its retention characteristics. In general, an analyte with a larger hydrophobic surface area (C- H, C-C, and generally non-polar atomic bonds, such as S-S and others) results in a longer retention time because it increases the molecule’s non-polar surface area, which is non- interacting with the water structure.
On the other hand, polar groups, such as -OH, -NH2, COO– or -NH3+ reduce retention as they are well integrated into water. Reversed phase HPLC has a non-polar stationary phase and an aqueous, moderately polar mobile phase. One common stationary phase is silica treated with RMe2SiCl, where R is a straight chain alkyl group such as C18H37 or C18H17. With these stationary phases, retention time is longer for molecules which are more non-polar, while polar molecules elute more readily.
Reversed phase chromatography has found both analytical and preparative applications in the area of biochemical separation and purification. Reversed phase chromatography is another very powerful technique and it is effective for the separation of a very wide range of molecules. However, at process scale it is not typically used for proteins, due to the presence of the organic solvent which denatures many proteins and destroys their biological activity. Reversed phase chromatography is used very frequently as an analytical technique and there are many different stationary phases available for method optimization.
Molecules that possess some degree of hydrophobic character, such as proteins, peptides and nucleic acids, can be separated by reversed phase chromatography with excellent recovery and resolution. In addition, the use of ion pairing modifiers in the mobile phase allows reversed phase chromatography of charged solutes such as fully deprotected oligonucleotides and hydrophilic peptides. Preparative reversed phase chromatography has found applications ranging from micropurification of protein fragments for sequencing to process scale purification of recombinant protein products.
G. Hydrophobic-Interaction Chromatography [HIC]:
It is a type of reversed-phase chromatography that is used to separate large biomolecules, such as proteins. It is usually desirable to maintain these molecules intact in an aqueous solution, avoiding contact with organic solvents or surfaces that might denature them. HIC takes advantage of the hydrophobic interaction of large molecules with a moderately hydrophobic stationary phase, e.g., butyl-bonded [C4], rather than octadecyl-bonded [C18], silica.
Initially, higher salt concentrations in water will encourage the proteins to be retained (salted out) on the packing. Gradient separations are typically run by decreasing salt concentration. In this way, biomolecules are eluted in order of increasing hydrophobicity.
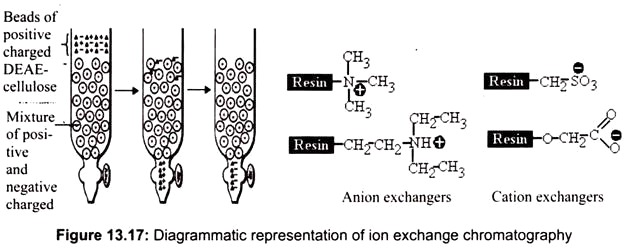
H. Ion Exchange Chromatography:
It is the most popular method for the purification of proteins and other charged molecules. It relies on charge-charge interactions between the proteins in sample and the charges immobilized on the resin of choice. It can be subdivided into cation exchange chromatography, in which positively charged ions bind to a negatively charged resin; and anion exchange chromatography, in which the binding ions are negative, and the immobilized functional group is positive.
Once the solutes are bound, the column is washed to equilibrate it in starting buffer, which should be of low ionic strength, and then the bound molecules are eluted off using a gradient of a second buffer which steadily increases the ionic strength of the eluent solution. Alternatively, the pH of the eluent buffer can be modified as to give the protein or the matrix a charge at which they will not interact and the molecule of interest elutes from the resin.
In cation exchange chromatography, raising the pH of the mobile phase buffer will cause the molecule to become less protonated and hence less positively charged. The result is that the protein no longer has the capability to form a strong ionic interaction with the negatively charged solid support which causes the molecule to elute from the chromatography column.
In anion exchange chromatography, lowering the pH of the mobile phase buffer will cause the molecule to become more protonated and hence more positively charged. The result is that the protein no longer has the capability to form a strong ionic interaction with the positively charged solid support which causes the molecule to elute from the chromatography column (Fig. 13.17).
Commonly used anion exchange resins are Q-resin (a Quaternary amine) and DEAE (Diethyl amino ethane) resin and cation exchange resins are S-resin (sulfate derivatives) and CM resins (carboxylate derived ions). Strong ion exchangers bear functional groups (e.g., quaternary amines or sulfonic acids) that are always ionized. They are typically used to retain and separate weak ions.
These weak ions may be eluted by displacement with a mobile phase containing ions that are more strongly attracted to the stationary phase sites. Alternately, weak ions may be retained on the column, then neutralized by in situ changing the pH of the mobile phase, causing them to lose their attraction and elute. Weak ion exchangers (e.g., with secondary-amine or carboxylic-acid functions) may be neutralized above or below a certain pH value and lose their ability to retain ions by charge.
I. Affinity Chromatography:
It is the most powerful technique which can potentially allow a one-step purification of the target molecule. In order to work, a specific ligand (a molecule which recognizes the target protein) must be immobilized on a support in such a way that allows it to bind to the target molecule. It is a chromatographic method of separating biochemical mixtures, based on a highly specific biologic interaction such as that between antigen and antibody, enzyme and substrate, or receptor and ligand. A classic example of this would be the use of an immobilized protein to capture its receptor (the reverse would also work). Affinity chromatography can be used in a number of applications, including nucleic acid purification, protein purification such as recombinant proteins from cell free extracts, and antibody purification from blood serum.
J. Size Exclusion Chromatography (SEC):
It is the separation of mixtures based on the molecular size of the components. Separation is achieved by the differential exclusion or inclusion, within the packing particles, of the sample molecules as they pass through a porous-particle stationary phase. The principle feature of SEC is its gentle non-adsorptive interaction with the sample, enabling high retention of biomolecular activity. For the separation of biomolecules in aqueous systems, SEC is referred to as gel filtration chromatography (GFC), while the separation of organic polymers in non-aqueous systems is called gel permeation chromatography (GPC).
Size exclusion chromatography is used primarily for analytical assays and semi- preparative purifications. It is typically not used for process scale work due to its lack of binding capacity.
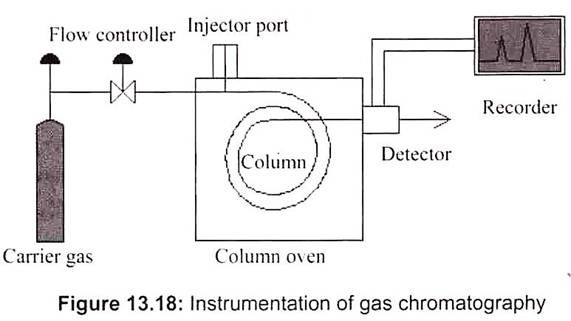
K. Gas Chromatography:
Specifically gas-liquid chromatography – involves a sample being vaporized and injected onto the head of the chromatographic column. The sample is transported through the column by the flow of inert, gaseous mobile phase. The column itself contains a liquid stationary phase which is adsorbed onto the surface of an inert solid. The stationary phase is a high-boiling liquid, which is packed into a long, narrow glass or metal column. The mixture to be analyzed is loaded by syringe into the beginning of this column. The mobile phase is an inert gas which continuously flows through the column. It is generally used for the study of essential oils present in plants.
The Gas Chromatography/Mass Spectrometry (GC/MS) instrument separates chemical mixtures (the GC component) and identifies the components at a molecular level (the MS component). The GC works on the principle that a mixture will separate into individual substances when heated. The heated gases are carried through a column with an inert gas (such as helium). As the separated substances emerge from the column opening, they flow into the MS. Mass spectrometry identifies compounds by the mass of analyte molecule (Fig. 13. 18).