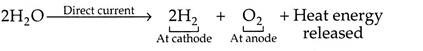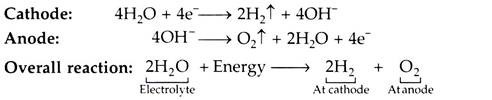Are you looking for an essay on ‘Hydrogen Energy’? Find paragraphs, long and short essays on ‘Hydrogen Energy’ especially written for school and college students.
Essay on Hydrogen Energy
Essay Contents:
- Essay on the Introduction to Hydrogen Energy
- Essay on the Properties of Hydrogen
- Essay on the Utilisation and Problems of Hydrogen Energy
- Essay on the Production of Hydrogen
- Essay on Hydrogen Storage
- Essay on Gas Hydrates
- Essay on Hydrogen Transportation
- Essay on the Advantages of Hydrogen as Fuel
- Essay on the Applications of Hydrogen Energy
1. Essay on Introduction to Hydrogen Energy:
Hydrogen energy (“energy carrier” like electricity) is an alternative energy of future which can also be stored in addition to other qualities of electrical energy. But it is highly inflammable and special handling precautions are needed during its production, transportation, storage and utilisation.
i. Hydrogen energy has a tremendous potential because it can be produced from water which is available in abundance in nature. It can be produced from water by using solar energy. All plants and hydrocarbons (fossil fuels) are sources of hydrogen.
ii. Hydrogen has the highest energy content per unit mass. Its specific energy content is almost three times that of hydrocarbon fuels. Therefore, it can be directly used as aircraft fuel for air transport. It has been used as a fuel for space crafts. A H2 – O2 fuel cell liberates energy and also water as sole material product for the use of space craft passengers.
iii. The simplest way to obtain hydrogen from water is its “electrolysis” using electricity. The latter can be generated from renewable energy sources like solar energy, wind energy and geothermal energy.
iv. The main issues associated with the use of hydrogen at energy source are:
(i) Production;
(ii) Storage and transportation;
(iii) Utilisation;
(iv) Safety and Management, and
(v) Economy.
v. One of the potential advantages of hydrogen as a secondary “fuel” is that it can be transmitted and distributed by pipeline in much the same way as natural gas. Hydrogen can serve as a means of carrying energy from the place where a primary source is available to a distant load centre where the energy is used.
vi. Hydrogen is an efficient and clean fuel. It has minimum carbon content compared to other fuels. A carbon-rich fuel produces more CO2 which contributes to global warming. By adopting a leaner carbon and richer hydrogen content, it is a step towards better “environmental friendly” source of fuel.
vii. Hydrogen has huge market, however, enormous capital investment is required for its production, distribution and storage. For the safe operation of equipment and systems, special design precautions are needed.
Sources of Hydrogen:
Hydrogen is found only in compound form with other elements. H2 combines with O2 to form water. Hydrogen combines with carbon to form different compounds such as coal, petroleum, methane gas etc.
2. Essay on the Properties of Hydrogen:
The properties of hydrogen are:
1. The burning process of hydrogen is pollution free.
2. The standard heating value of hydrogen gas is 12.1 MJ/m3 compared with 38.3 MJ/m3 for natural gas.
3. The heating value of liquid hydrogen is 120 MJ/kg or 8400 MJ/m3 as compared to 44 MJ/kg or 32000 MJ/m3 of aviation petrol.
4. Hydrogen is a light gas at room temperature and pressure. Its density is 1/4th of that air and 1/9th that natural gas.
5. Hydrogen can be liquefied at -253°C at atmospheric pressure. The liquid hydrogen has a specific gravity of 0.07 which is 1/10th that of gasoline.
6. Mixture of hydrogen and air are combustible over wide range of composition. The flammability limits are from 4 to 74% by volume of hydrogen in air at ordinary temperatures.
7. The flame speed of hydrogen when burning in air is much greater than for natural gas.
8. The ignition energy to initiate combustion is less for hydrogen than for natural gas.
9. The combustion of hydrogen with oxygen from air results in release of energy and water as byproduct.
10. Detonation can occur between hydrogen-air mixture between 18 and 59%. The I.C. engines working on hydrogen fuel can work from very rich (excess fuel) to very lean (excess air) mixture. The adjustment of air-fuel ratio is less critical than for gasoline engine.
3. Essay on the Utilisation and Problems of Hydrogen Energy:
Following are the possible areas of utilisation of hydrogen in the near future:
1. Use of hydrogen in the processing of heavy oil.
2. Reduction of iron oxides by means of hydrogen in the steel industry.
3. Using hydrogen to manufacture synthetic liquid or gaseous fuels.
4. Direct addition of hydrogen to the existing natural gas distribution network.
5. Direct use of hydrogen as an aircraft fuel in air transport.
6. Direct use of hydrogen as a motor vehicle fuel in urban transport, particularly where air pollution problems are already critical.
7. Production of electrolytic hydrogen, for full-load exploitation of nuclear power stations.
The use of hydrogen entails the following problems:
1. Commercial production of hydrogen at cheap cost.
2. Effective energy utilisation.
3. Difficulty in storage, since it is highly explosive.
4. Lack of safety and management.
4. Essay on the Production of Hydrogen:
The most commonly used methods of production of hydrogen are enumerated and described as follows:
1. Electrolysis of Water:
It is the process in which hydrogen splits from water by means of direct electrical current, by using two electrodes and electrolyte.
The reaction of electrolysis is as follows:
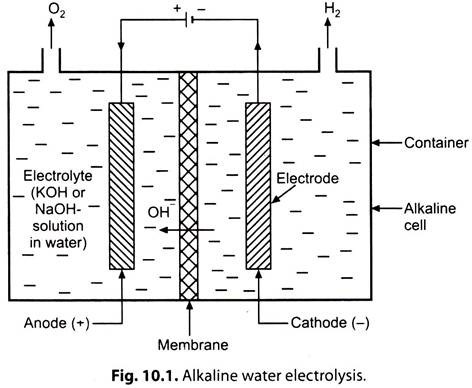
Fig. 10.1 shows the principle of operation of an alkaline water electrolysis cell.
When the direct current flows through the electrolyte from the anode (+ve electrode), to the cathode (-ve electrode), the water in the electrolyte solution is decomposed into H2 which is released at the cathode, and 02 released at the anode. Although only the water is split, an electrolyte (e.g. KOH solution) is required because water itself is a very poor conductor of electricity.
The reactions at cathode and anode are given below:
The energy required to produce hydrogen is 3.5 kWh/m3.
The available electrolysis processes are:
1. Alkaline electrolysis,
2. Membrane electrolysis, and
3. High temperature steam electrolysis.
2. Steam Reformation (Fossil Fuels):
In this process, steam is passed over hot sponge iron sheets at a suitable temperature where hot iron and steam react to produce ferric oxide (Fe2 O3), hydrogen (H2), carbon dioxide (CO2) and carbon monoxide (CO) in small quantities. The gases are passed through a scrubber where dilute NaOH absorbs CO2 and CO.
Reaction- 3H2O + 2Fe Fe2O3 + 3H2
3. Coal Gasification:
In the gasification of coal, there is complete conversion of the organic part of the coal into gas, so that ash alone remains. This is done by reacting the coal with a gasifying agent e.g. steam above 700°C.
In this process, the carbon in coal reacts with steam to form CO and H2. This low energy gas mixture is submitted to water gas shift reaction with steam. The CO is then converted into CO2 with the formation of additional H2 gas.
The reactions for coal gasification are:
C + H2O —> CO + H2
CO + H2O + H2 —> 2H2 + CO2
4. Methane Gas Reformation:
In this process, methane mixed with steam is passed over a nickel oxide catalyst at elevated temperature. The reforming reaction being endothermic, it is usually carried out in fire tube reformed in a fire to be reformer where the catalyst is loaded in the tubes.
The reaction for methane gas reforming:
CH4 + H2O —> CO + 3H2 + Heat energy released.
After steam reforming, the gas products contain considerable amount of CO which may further undergo reaction with additional steam, and it increases the H2 production.
The reaction of CO with steam:
CO + H2O —> CO2 + H2 + Heat energy absorbed.
The above reaction is known as water gas shift reaction which is exothermic.
5. Thermo-Chemical Method:
The thermo-chemical method involves thermal chemical reactions between primary energy, water and specific chemicals to produce hydrogen at temperatures range from 700°C to 1000°C.
General thermo-chemical reactions:
ZOx + H2O —> ZOx+1 + H2
ZOx+1 + Heat —> ZOx + ½O2
where Z represents a metallic ion or a complex radical.
6. Solar Energy Methods:
These methods are described below:
a. Bio-Photolysis (Biological Production):
In this process, Hydrogen is produced with the help of photosynthetic microorganisms. Blue green algae as well as other anaerobic bacteria are capable of splitting water into hydrogen and oxygen by light driven process and such a process is called bio- photolysis.
b. Photo-Electrolysis:
In this process, the decomposition of water into hydrogen and oxygen takes place with the help of electric current which is generated by exposing electrode to sunlight.
In this process at least one of the electrodes is usually semiconductor; a catalyst may be included to facilitate the electrode process.
5. Essay on Hydrogen Storage:
The need for storage arises due to the almost inevitable mismatch between the optimum production rate of energy and fluctuations in demand for energy by users.
The following three methods are used for storage of hydrogen:
1. Compressed Gas Storage:
Hydrogen can be stored in compressed gaseous state in underground reservoirs similar to natural gas or can be stored in high pressure cylinders.
This method of storage is costly as large quantity of steel is required to store a small amount of hydrogen. The gaseous storage of hydrogen, for industrial use is economically not viable as a fuel.
2. Liquid Storage:
Liquid hydrogen fuel is used as a rocket propellent in space vehicles as it has the highest energy density which is almost three times the conventional fuels.
It boils at -253°C (i.e., 20 K) and therefore must be maintained at or below this temperature in storage unless pressure build up can be tolerated. It is necessary to use insulated cylinder to avoid air condensation over its surface.
The main problems associated with the storage of hydrogen are:
1. Flammabily danger from the fact that liquefied atmospheric gases, rich in oxygen, would concentrate in the vicinity of hydrogen tank.
2. Considerable amount of energy (25-30% of the heating value of hydrogen) is required to convert hydrogen gas into the liquid phase. Thus, a liquid hydrogen plant requires some kind of primary refrigeration, such as a liquid nitrogen plant, to precool hydrogen.
3. Solid State Storage:
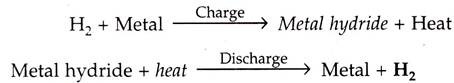
A number of metals and alloys form solid compounds, called metal “hydrides”, by direct reaction with hydrogen gas.
In a solid storage, the hydrogen is stored in the form of metallic hydrides. The metal hydride system is based on the principle that a few metals absorb hydrogen in an ‘exthermonic reaction’ when treated with gas and the absorbed gas is released when the metal hydride is heated.
The chemical reactions given below:
For hydrogen storage, the metal hydride should have the following properties:
1. Large amount of hydrogen per unit volume per unit mass.
2. Fairly inexpensive.
3. Release of gas at a significant pressure from the hybrid at a moderately high temperature, preferably below 100°C.
4. Easy formation of hybride (by reaction of metal with H2 gas) should be stable at room temperature.
6. Essay on Gas Hydrates:
Gas hydrates have been identified by ONGC (Oil Natural Gas Commission) as one of the non-conventional energy sources, to be studied for exploration to ensure energy security for the country. These are naturally occurring ice like compounds of methane and are water formed under low temperature and pressure conditions. These ice formations consist of water molecules that trapped gas molecules in a cage-like structure, found at varying depths in areas of low temperature.
The gas hydrates are important due to following reasons:
1. Total energy contained in hydrogen is estimated to be double the amount of the total fossil fuels.
2. Contain a great volume of methane, which is a source of cleaner fuel.
3. One volume of gas hydrates produces 164 volume of gas at standard temperature and pressure.
4. Methane made by drilling around these gas hydrates, can be captured, stored and fed into pipelines for further use.
Methane can be used to extract hydrogen and use it to power fuel cells.
7. Essay on Hydrogen Transportation:
Hydrogen can be transported by the following three methods:
1. By Pipelines:
Long distance gas transmission lines of lengths greater than about 90 km must be supplied with pipeline compressors at fairly regular intervals. These compressors must handle a considerably greater volume of the gas-somewhere between 3 to 4 times the number of m3 to the same energy capacity. This would require a considerably higher horse power to drive a hydrogen compressor (in comparison then that needed to drive a natural gas compressor for the same energy throughout).
Therefore, the cost of hydrogen transportation by pipelines must include the cost of piping, compressors and power consumption by compressors.
2. By Insulated Tanks:
Hydrogen in bulk can be transported in well insulated cryogenic tanks having liquid hydrogen either by trucks or rails. The transportation cost, however, is very high.
3. By Metal Hydrides:
Hydrogen can also be transported as a solid metal hydride. The main drawback is the heavy weight of hydride relative to its hydrogen yield. The transportation cost is very high.
Safety Precautions:
Hydrogen is highly inflammable and explosive and can lead to fire and serious accidents.
The production, storage and distribution of hydrogen require special precautions, as given below:
1. The system should be designed to withstand the explosion pressures.
2. The system should be designed to withstand pressure surges.
3. Proper explosion relief system must be provided.
4. Flame traps, flame suppressors, explosion-relief devices and rapid closing devices must be used.
5. The design, manufacture and storage methods/system should follow Petroleum Act.
8. Essay on the Advantages of Hydrogen as Fuel:
Following are the advantages of hydrogen as fuel:
1. Very high energy content.
2. Burning is non-polluting.
3. Hydrogen produced from biomass and supplied to the consumers in the transport sector costs only 50% compared to hydrogen produced electrolytically.
4. For fuel-cell operated bus, hydrogen produced from biomass can compete well with gasoline-operated vehicles.
5. It is a superior fuel for turbojet aircraft due to greater economy, lower noise level and little pollution.
6. Hydrogen as a velicular fuel can reduce dependence on fossil fuel which is increasing in cost every year.
7. Hydrogen can easily be transported and distributed through pipelines.
8. Hydrogen being a high density fuel, its low transport cost compensates for its high product cost to make it an economically viable fuel.
9. Hydrogen can be used for generating electricity for domestic appliances, in domestic cooking as a fuel, in automobiles etc.
9. Essay on the Applications of Hydrogen Energy:
Following are the applications of hydrogen energy:
1. It can be used for H2 -O2fuel cell for production of electrical energy.
2. Hydrogen is used as fuel in aircrafts and rockets in liquid form.
3. Used in cooking, water heaters and air-conditioning.
4. Can also be used in furnaces.
5. Can also be used in generators.
6. Widely used in petroleum refining.
7. Widely used in manufacture of vanaspati, fertilizers and alcohols.
i. Hydrogen-based vehicles have been developed by Mazda Motor Corporation, BMW Germany, Toyota hybrid highlander and Taiwanese scooter. In India, Tatas are working on the modification of I.C. engines in the present vehicles that can be run on hydrogen fuel.
ii. National Hydrogen Energy Board (NHEB) has prepared a workable plan to make hydrogen as a commercial fuel. In the near future, large amounts of hydrogen could be produced in remote wind farms, solar stations and ocean power plants, and stored underground.