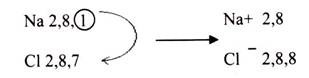Chemical Bonds Essay – This is one of the best essays on ‘Chemical Bonds and Its Types’ especially written for school and college students.
Essay # 1. Introduction to Chemical Bonds:
Chemical compounds are formed by the joining of two or more atoms. The attractive force which keeps atoms together is known as chemical bond. The bonds present between atoms give the molecules different properties. A stable compound occurs when the total energy of the combination has lower energy than the separated atoms.
The more charges a positive ion has, the greater the attraction towards its accompanying negative ion. The greater the attraction, the more energy is released when the ions come together. Energy is needed to remove electrons from atoms. This is called ionization energy. The total ionization energy increases as the electrons are removed.
Essay # 2. Types of Chemical Bonds:
The types of bonds present between the atoms can be:
i. Ionic Bonds:
In ionic bonding, electrons are completely transferred from one atom to another. In the process of either losing or gaining negatively charged electrons, the reacting atoms form ions. The oppositely charged ions are attracted to each other by electrostatic forces, which are the basis of the ionic bond. For example, during the reaction of sodium with chlorine: sodium loses its one valence electron to chlorine, resulting in a positively charged sodium ion and a negatively charged chlorine ion. If a sodium atom gives an electron to a chlorine atom, both become more stable.
The sodium has lost an electron, so it no longer has equal number of electrons and protons; it has a charge of+1 since there is one more proton than electron. If electrons are lost from an atom then positive ions are formed, which are called cations.
The chlorine has gained an electron, so it now has one more electron than proton. It therefore has a charge of -1. If electrons are gained by an atom then negative ion is formed, called an anion.
ii. Covalent Bonds:
A covalent bond is a bond that is formed when two atoms share electrons. A single bond is formed when 1 pair of electrons is shared; a double bond is formed when 2 pairs of electrons are shared; and a triple bond is formed when 3 pairs of electrons are shared. Bonding between non-metals consists of two electrons shared between two atoms.
The covalent bond involves an overlap of the electron clouds from each atom. The electrons are concentrated in the region between the two atoms. In covalent bonding, the two electrons shared by the atoms are attracted to the nucleus of both atoms. Neither atom completely loses or gains electrons as in ionic bonding.
There are two types of covalent bonding:
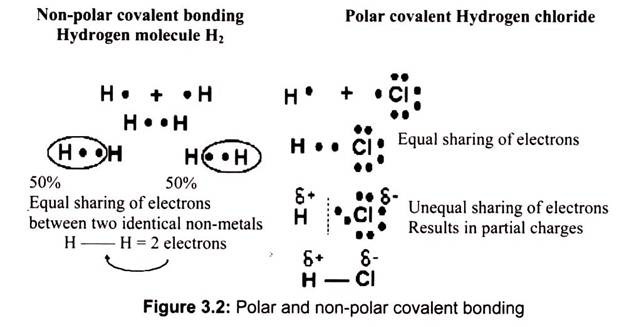
(a) Non-Polar Bonding:
It is by equal sharing of electrons. The simplest non-polar covalent molecule is hydrogen. Each hydrogen atom has one electron and needs two to complete its first energy level. Since both hydrogen atoms are identical, neither of the atoms will be able to dominate in the control of the electrons. The electrons are therefore shared equally.
(b) Polar Bonding:
It is an unequal sharing of electrons. The number of shared electrons depends on the number of electrons needed to complete the octet. Hydrogen chloride forms a polar covalent molecule. The chlorine has 7 and hydrogen has 1 electron in its outer energy shell. Since 8 electrons are needed for an octet, they share the electrons.
However, chlorine gets an unequal share of the two electrons, although the electrons are still shared (not transferred as in ionic bonding), it is unequal (Fig. 3.2). The electrons spend more of the time closer to chlorine. As a result, the chlorine acquires a “partial” negative charge. At the same time, since hydrogen loses the electron most but not all of the time, it acquires a “partial” positive charge. The partial charge is denoted with a small Greek symbol for delta.
iii. Hydrogen Bonds:
A hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule. Usually, the electronegative atom is oxygen, nitrogen, or fluorine, which has a partial negative charge.
The hydrogen then has the partial positive charge. The hydrogen bond is much weaker than both the ionic bond and the covalent bond. Within macromolecules such as proteins and nucleic acids, it can exist between two parts of the same molecule and figures as an important constraint on such molecules’ overall shape.
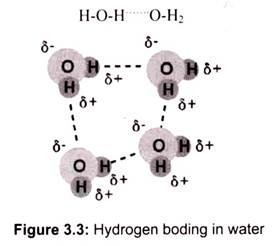
The most ubiquitous and perhaps simplest example of a hydrogen bond is found among the water molecules (Fig. 3.3). In a discrete water molecule, water has two hydrogen and one oxygen atom. Two molecules of water can form a hydrogen bond between them.
The oxygen of one water molecule has two lone pairs of electrons, each of which can form a hydrogen bond with hydrogens on two other water molecules. This can repeat so that every water molecule is H-bonded with four other molecules (two through its two lone pairs and two through its two hydrogen atoms).
The high boiling point of water is due to the high number of hydrogen bonds present in it. Water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is four.
Hydrogen bonding also plays an important role in determining the three-dimensional structures adopted by proteins and nucleic acids. In these macromolecules, bonding between parts of the same macromolecule helps to fold into a specific shape, which helps in determining physiological or biochemical role of the molecules. For example the double helical structure of DNA is mainly due to hydrogen bonding between the base pairs, which link one complementary strand to the other and hence enable replication.