Are you looking for an essay on ‘Metabolism’? Find paragraphs, long and short essays on ‘Metabolism’ especially written for school and college students.
1. Essay on the Introduction to Metabolism:
The term metabolism is defined as ‘the chemical processes by which nutritive material is built up into living matter, or by which complex molecules are broken down into simpler substances during the performance of special functions’. The various reactions which involve the synthesis of complex molecules are grouped under anabolism, whereas the breakdown of complex molecules is known as catabolism.
Both anabolic and catabolic processes include a vast number of different chemical reactions, but there are number of common features. Most of the metabolic processes occur inside the cells of the body, mainly in the cytoplasm, but also inside intracellular organelles such as the mitochondria. Anabolic and catabolic reactions involve the action of enzymes and the utilization of energy.
Metabolism, a vital process for all life forms, is a constant process that begins when an organism being conceived and ends when it dies. In case the metabolism stops, results in death. The process of metabolism is really a balancing act involving two kinds of activities that go on at the same time the building up of body tissues and energy stores (anabolism or constructive metabolism) and the breaking down of energy stores to generate more fuel for body functions (catabolism or destructive metabolism).
Almost all of the chemical reactions in the living body require the expenditure of energy, which is made available mainly by the catabolism of the ‘macronutrients’ fats and carbohydrates (particularly glucose), and proteins (to a small extent). According to the law of conservation of energy, the total energy of a system remains constant, though energy may transform into another form. In the body’s metabolism, the energy released from the oxidation of the macronutrients is used for a series of chemical reactions, instead of being released only as heat.
A fundamental feature of both anabolic and catabolic processes is the utilization of energy. The ultimate source of energy for all living system is solar energy. Thus, the metabolic process on earth begins with the producers, the plants. First, a green plant takes energy from sunlight. The plant uses this energy and the molecule chlorophyll (which gives plants their green color) to build sugars from water and carbon dioxide in a process known as photosynthesis.
The men and the animals when eat the plants, they take this energy (in the form of sugar), along with other vital cell-building chemicals. The body’s next step is to break the sugar down so that the energy released can be distributed to, and used as fuel by the body’s cells. These reactions are made easy by biological catalysts, (enzymes) and they break down proteins into amino acids, fats into fatty acids and carbohydrates into simple sugars (e.g., glucose).
During these processes, the energy from these compounds can be released by the body for use or stored in body tissues, especially the liver, muscles, and body fat. During anabolism, small molecules are changed into larger, more complex molecules of carbohydrate, protein and fat.
2. Essay on Carbohydrate Metabolism:
In animals, especially in human the major source of dietary carbohydrate is starch from consumed plant material and a small amount of glycogen from animal tissue as well as disaccharides such as sucrose from products containing refined sugar and lactose in milk. Digestion in the gut converts all carbohydrate to monosaccharides which are transported to the liver and converted to glucose. The liver has a central role in the storage and distribution within the body of all fuels, including glucose.
Carbohydrate metabolism begins with digestion in the small intestine; here monosaccharides are absorbed into the blood stream.
Blood sugar concentrations are controlled by three hormones: .
a. Insulin,
b. Glucagon, and
c. Epinephrine.
When the concentration of glucose in the blood increases, insulin is secreted by the pancreas, which stimulates the transfer of glucose into the cells, especially in the liver and muscles, although other organs are also able to metabolize glucose.
Glucose in the body undergoes catabolism in all peripheral tissues, particularly in brain, muscle and kidney to produce ATP. Excess glucose is changed into glycogen by the process of glycogenesis (anabolism) and stored as glycogen in liver and muscle or converted to fatty acids and is stored in adipose tissue as triglycerides. Eqinephrine and glucagon hormones are secreted to stimulate the conversion of glycogen to glucose when blood glucose level becomes low. This process is called glycogenolysis (catabolism).
Glucose metabolism begins with the process called glycolysis (catabolism). The end products of glycolysis are pyruvic acid and ATP. Since glycolysis releases relatively little ATP, further reactions continue to convert pyruvic acid to acetyl CoA and then citric acid in the citric acid cycle. The majority of the ATPs are made from oxidations in the citric acid cycle in connection with the electron transport chain. During strenuous muscular activity, pyruvic acid is converted into lactic acid rather than acetyl CoA. During the resting period, the lactic acid is converted back to pyruvic acid. The pyruvic acid in turn is converted back to glucose by the process called gluconeogenesis (anabolism).
3. Essay on Glycolysis (Catabolism):
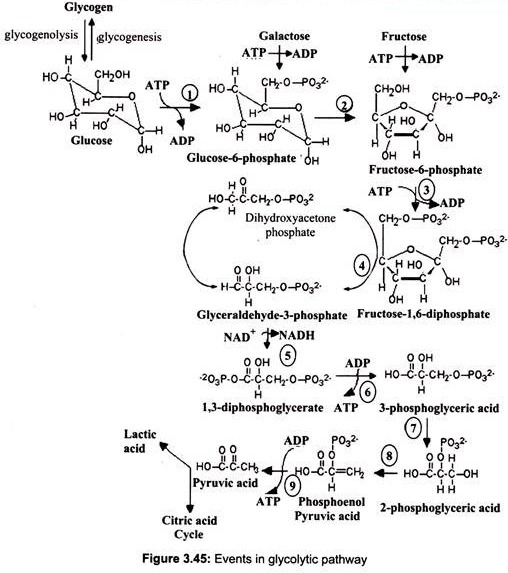
Glycolysis (Embden-Meyerhof pathway) is the initial metabolic pathway of carbohydrate catabolism. It is the most universal process by which cells of all types derive energy from sugars. Glucose is oxidized by all tissues to synthesize ATP. The first pathway which begins the complete oxidation of glucose is called glycolysis. This pathway cleaves the six carbon glucose molecule (C6H12O6) into two molecules of the three carbon compound pyruvate (C3H3O3–). This oxidation is coupled to the net production of two molecules of ATP per glucose. Glycolysis converts one molecule of glucose into two molecules of pyruvate, along with “reducing equivalents” in the form of the coenzyme NADH.
The global reaction of glycolysis is:
Glucose + 2 NAD+ + 2 ADP + 2 Pi –> 2 NADH+ 2 pyruvate + 2 ATP + 2 H2O + 4 H+
In eukaryotes, glycolysis takes place within the of the cell. Glucose gets into the cell through facilitated diffusion. The first step in glycolysis is phosphorylation of glucose by hexokinase (in liver the most important hexokinase is glucokinase). This reaction consumes 1 ATP molecule. Although the cell membrane is permeable to glucose because of the presence of glucose transport proteins, it is impermeable to glucose 6-phosphate.
Glucose 6- phosphate is then rearranged into fructose 6-phosphate by phospho-glucose isomerase. (Fructose can also enter the glycolytic pathway at this point.). Phosphofructokinase-1 then consumes 1 ATP to form fructose 1, 6-bisphosphate. The energy expenditure in this step is justified in 2 ways- the glycolytic process is now irreversible, and the energy supplied to the molecule allows the ring to be split by aldolase into 2 molecules – dihydroxyacetone phosphate and glyceraldehyde 3-phosphate. (Triosephosphate isomerase converts the molecule of di-hydroxy-acetone phosphate into a molecule of glyceraldehyde 3-phosphate.) Each molecule of glyceraldehyde 3-phosphate is then oxidized by a molecule of NAD+ in the presence of glyceraldehyde 3-phosphate dehydrogenase, forming 1, 3-bisphosphoglycerate.
Phosphoglycerate kinase then generates a molecule of ATP while forming 3- phosphoglycerate. At this step, glycolysis has reached the break-even point- 2 molecules of ATP were consumed and 2 new molecules have been synthesized. Phosphoglyceromutase then forms 2-phosphoglycerate; enolase then forms phosphoenolpyruvate and another sub- strate-level phosphorylation later forms a molecule of pyruvate and a molecule of ATP by means of the enzyme pyruvate kinase (Fig. 3.45).
NAD is used as the electron acceptor in the oxidation reaction. This cofactor is present only in limited amounts and once reduced to NADH, as in this reaction, it must be reoxidised to NAD to permit continuation of the pathway.
Methods of Glycolysis:
This re-oxidation occurs by one of two methods:
(i) Anaerobic Glycolysis:
In the absence of oxygen, pyruvate is reduced to lactate that is ideally suited to utilization in heavily exercising muscles where oxygen supply is often insufficient to meet the demands of aerobic metabolism. The reduction of pyruvate to lactate is coupled to the oxidation of NADH to NAD.
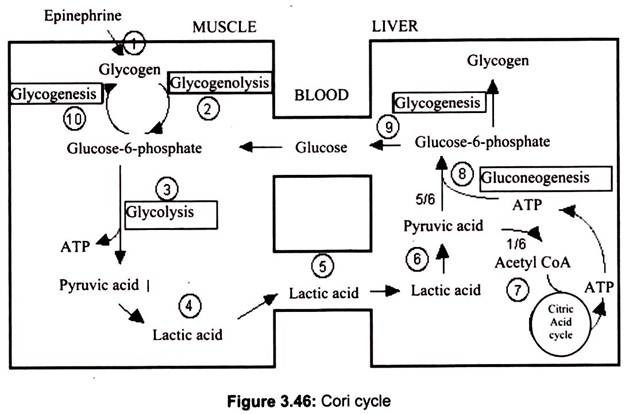
Cori Cycle:
The lactate formed is transported to other tissues and dealt with by one of the two mechanisms such as converted back to pyruvate or converted back to glucose in the liver. The process of conversion of lactate to glucose is called gluconeogenesis, uses some of the reactions of glycolysis (but in the reverse direction) and some reactions unique to this pathway to re-synthesize glucose.
The majority of the enzymes responsible for gluconeogenesis are found in the cytoplasm; the exception is pyruvate carboxylase which is located in the mitochondria. This pathway requires ATP but has the role of maintaining a circulating glucose concentration in the bloodstream (even in the absence of dietary supply) and also maintaining a glucose supply to fast twitch muscle fibres.
The Cori cycle, named after its discoverers, Carl Cori and Gerty Cori, refers to the metabolic pathway in which lactate produced by anaerobic glycolysis in the muscles moves to the liver and is converted to glucose, which then returns to the muscles and is converted back to lactate (Fig. 3.46). It can be shown by a complex calculation of energy yields that this process of partially oxidizing glucose to lactate in muscle, transporting it to the liver for conversion back to glucose and then re-supplying it to muscle, actually has a much higher energy yield than the 2 ATP/glucose produced by glycolysis alone.
(ii) Aerobic Glycolysis:
In aerobic condition pyruvate is transported inside mitochondria and oxidized to acetyl coenzyme A (abbreviated to “acetyl CoA“). This is an oxidation reaction and uses NAD as an electron acceptor. Further, acetyl CoA is oxidized ultimately to CO2 by citric acid cycle. These reactions are coupled to a process known as the electron transport chain which has the role of harnessing chemical bond energy through a series of oxidation/reduction reactions to the synthesis of ATP and simultaneously re-oxidizing NADH to NAD.
(a) Citric Acid Cycle:
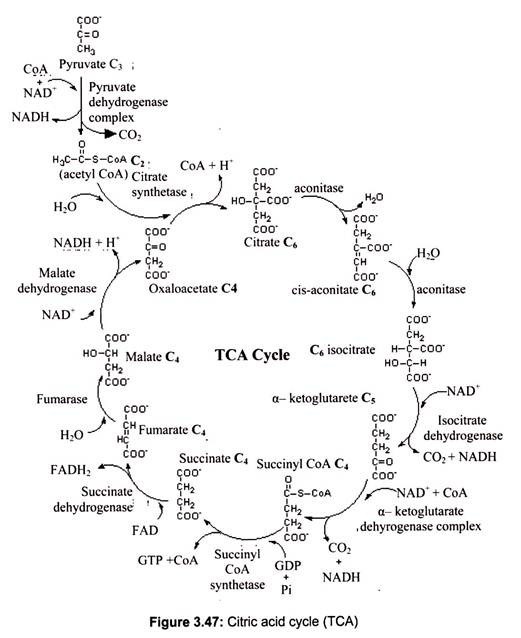
The Krebs cycle, also known as the tri-carboxylic acid cycle (TCA), was first recognized in 1937 by the man for whom it is named, German biochemist Hans Adolph Krebs, the winner of Nobel Prize in 1953. In short, the Krebs cycle constitutes the discovery of the major source of energy in all living organisms. The Krebs cycle reactions take place in the matrix of the mitochondria. Some of the final steps of intermediate metabolism take place there, as well.
For example, in the matrix as well as the cytoplasm, glutamate (the amino acid glutamic acid) loses its amino group and is oxidized to alpha-ketoglutarate. Under aerobic conditions the end product of glycolysis is pyruvic acid converted to acetyl coenzyme A (acetyl CoA) which is the initiator of the citric acid cycle. In carbohydrate metabolism, acetyl CoA is the link between glycolysis and the citric acid cycle. The citric acid cycle contains the final oxidation reactions, coupled to the electron transport chain, which produce the majority of the ATP in the body.
For each glucose molecule that enters glycolysis, two pyruvate molecules are produced and have gained two NADH and two ATPs, while in the Calvin cycle approximately 54 ATPs are utilized by the plant to synthesize one glucose molecule. ATP is generated by breaking the bonds in glucose and capturing as much as possible of the energy stored in that molecule. The CA cycle produces very little ATP directly, but generates many molecules of reduced coenzymes NAD and FAD as NADH and FADH2.
The Krebs cycle begins with oxalo-acetate and combines with Acetyl CoA to cycle through one complete turn. After Acetyl CoA is oxidized to CO2 and H2O, the electrons drive proton pumps which generate ATP that is greatly needed by the cell. Remember that the NADH molecules are important because they contain extracted electrons which ultimately reduce NAD+.
However, when the electrons do not have enough energy to reduce NAD+, they are stored temporarily in the FADH2 molecule. Each NADH molecule is responsible for the production of three ATP molecules, while FADH2 is responsible for the production of two ATP molecules. In prokaryotic cells, the citric acid cycle occurs in the cytoplasm; in eukaryotic cells the citric acid cycle takes place in the matrix of the mitochondria.
The overall reaction for the citric acid cycle is:
2 acetyl groups + 6 NAD+ + 2 FAD + 2 ADP + 2 Pi –> 4 CO2 + 6 NADH + 6H+ + 2 FADH2 + 2 ATP
The citric acid cycle provides a series of intermediate compounds that donate protons and electrons to the electron transport chain by way of the reduced coenzymes NADH and FADH2. The electron transport chain then generates additional ATPs by oxidative phosphorylation.
The TCA cycle involves 8 distinct steps, each catalyzed by a unique enzyme:
i. The citric acid cycle begins when Coenzyme A transfers its 2-carbon acetyl group to the 4- carbon compound, oxalo-acetate, to form the 6-carbon molecule, citrate.
ii. The citrate is rearranged to form an isomeric form, isocitrate (Fig. 3.47).
iii. The 6-carbon isocitrate is oxidized and a molecule of CO2 is removed producing the 5- carbon molecule α-ketoglutarate. During this oxidation, NAD+ is reduced to NADH + H+.
iv. Alpha-ketoglutarate is oxidized, carbon dioxide is removed, and coenzyme A is added to form the 4-carbon compound succinyl-CoA. During this oxidation, NAD+ is reduced to NADH + H+
v. CoA is removed from succinyl-CoA to produce succinate. The energy released is used to make guanosine triphosphate (GTP) from guanosine diphosphate (GDP) and Pi by sub- strate-level phosphorylation. GTP can then be used to make ATP.
vi. Succinate is oxidized to fumarate. During this oxidation, FAD is reduced to FADH2.
vii. Water is added to fumarate to form malate.
viii. Malate is oxidized to produce oxaloacetate, the starting compound of the citric acid cycle. During this oxidation, NAD+ is reduced to NADH + H+
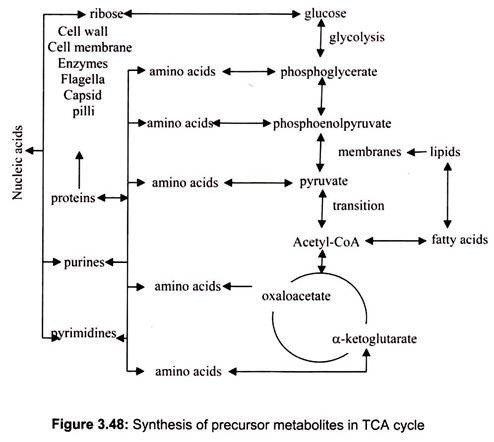
In addition to their roles in generating ATP by catabolism, the citric acid cycle also supplies precursor metabolites (anabolic) for various biosynthetic pathways (Fig. 3.48).
(b) Electron Transport Chain:
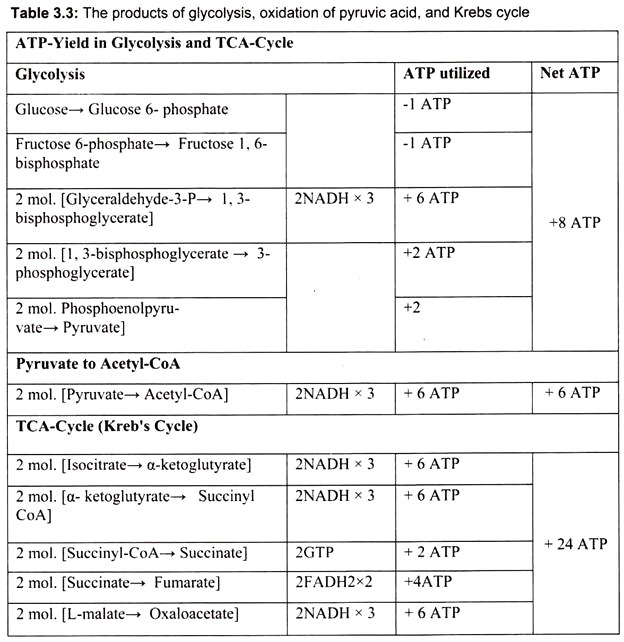
It is the final part of the phase-II of aerobic respiration. In respiration, oxidation of the substrate occurs by dehydrogenation (i.e., removal) of hydrogen atoms (2H) from the substrate. Most of these hydrogen atoms are accepted by NAD to form reduced co-enzyme NADH. In the aerobic respiration 10 NADH2 are formed (2NADH2 in glycolysis + 8 NADH2 in Krebs cycle) from one molecule of glucose. Also, in Krebs cycle, hydrogen is accepted by FAD to form FADH2 in one step; a total of 2 FADH2 are formed by aerobic respiration of each glucose molecule.
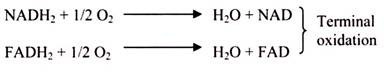
Each molecule of reduced co-enzyme thus formed in aerobic respiration (glycolysis and Krebs cycle) is finally oxidized by the free molecular oxygen through a process called terminal oxidation (Fig. 3.49).
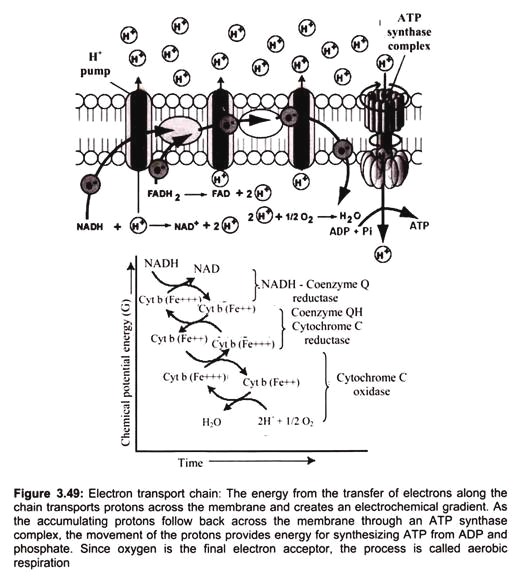
The respiratory chain (or the ETS) is present in the inner membrane of mitochondrion (i.e., in the cristae membrane). It consists of various enzymes and co-enzymes which act as electron carriers. Embedded in the inner membrane are proteins and complexes of molecules that are involved in the process called electron transport. The electron transport system (ETS), as it is called, accepts energy from carriers in the matrix and stores it to a form that can be used to phosphorylate ADP.
Two energy carriers are known to donate energy to the ETS, namely nicotine adenine di-nucleotide (NAD) and flavin adenine di-nucleotide (FAD). NADH binds to complex -I. It binds to a prosthetic group called flavin mononucleotide (FMN), and is immediately re-oxidized to NAD. NAD is recycled, acting as an energy shuttle. FMN receives the hydrogen from the NADH and two electrons. It also picks up a proton from the matrix. In this reduced form, it passes the electrons to iron-sulfur clusters that are part of the complex, and forces two protons into the inter-membrane space. Reduced NAD carries energy to complex I (NADH-Coenzyme Q Reductase) of the electron transport chain. FAD is a bound part of the succinate dehydrogenase complex (complex II).
Electrons cannot pass through complex-l without accomplishing proton translocation. Electron transport carriers are specific, in which each carrier accepts electrons (and associated free energy) from a specific type of preceding carrier. Electrons pass from complex I to a carrier (Coenzyme Q) embedded by itself in the membrane. From Coenzyme Q electrons are passed to a complex -III which is associated with another proton translocation event.
Complex-II, the succinate dehydrogenase complex, is a separate starting point, and is not a part of the NADH pathway. From succinate, the sequence is Complex II to Coenzyme Q to Complex III to cytochrome C to Complex IV. Thus, there is a common electron transport pathway beyond the entry point, either Complex I or Complex II. Protons are not translocated at Complex II. There is not sufficient free energy available from the succinate dehydrogenase reaction to reduce NAD or to pump protons at more than two sites. From Complex III the pathway moves to cytochrome C then to a Complex IV (cytochrome oxidase complex). More protons are translocated by Complex IV, and it is at this site that oxygen binds, along with protons, and using the electron pair and remaining free energy, oxygen is reduced to water.
Oxygen serves as an electron acceptor, clearing the way for carriers in the sequence to be re-oxidized so that electron transport can continue. The purpose of electron transport is to conserve energy in the form of a chemiosmotic gradient. The gradient, in turn, can be exploited for the phosphorylation of ADP as well as for other purposes. With the cessation of aerobic metabolism cell is damaged immediately and irreversibly.