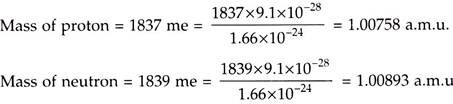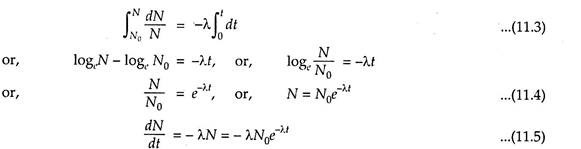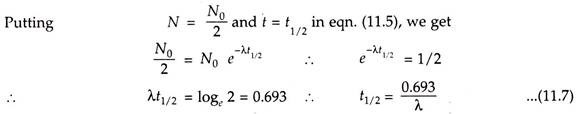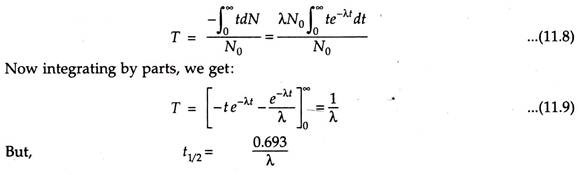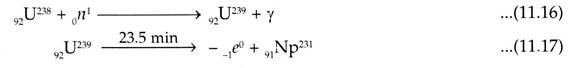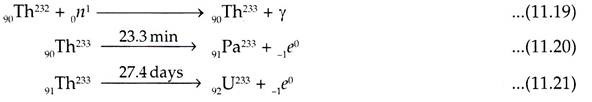Are you looking for an essay on the ‘Aspects of Nuclear Engineering’? Find paragraphs, long and short essays on the ‘Aspects of Nuclear Engineering’ especially written for school and college students.
Essay on Nuclear Engineering
Essay Contents:
- Essay on the Atomic Structure of an Element
- Essay on Atomic Mass Unit
- Essay on Isotopes
- Essay on Radioactivity
- Essay on Radioactive Decay of an Element
- Essay on Nuclear Reactions within an Atom
- Essay on Fertile Materials
- Essay on the Fission and Fusion of Neutrons
1. Essay on the Atomic Structure of an Element:
i. An element is defined as a substance which cannot be decomposed into other substances. The smallest particle of an element which takes part in chemical reaction is known as an ‘atom’. The word atom is derived from Greek word ‘Atom’ which means indivisible and for a long time the atom was considered as such.
Dalton’s atomic theory states that:
(a) all the atoms of one element are precisely alike, have the same mass but differs from the atoms of other elements,
(b) the chemical combination consists of the union of a small fixed number of atoms of one element with a small fixed number of other elements.
ii. Various atomic models proposed by scientists over the last few decades are:
1. Thompson’s plum puddling model,
2. Rutherford’s nuclear model,
3. Bohr’s model,
4. Sommerfeld’s model,
5. Vector model,
6. Wave-mechanical model.
iii. The complex structure of atom can be classified into electrons and nucleus. The nucleus consists of protons and neutrons both being referred as nucleons. Protons are positively charged and neutrons are neutral, thus making complete nucleus as positively charged.
iv. The electrons carry negative charge and circulate about the nucleus. As the positive charge on proton particle is equal to the negative charge on electron particle, and the number of electrons is equal to the number of protons, atom is a neutral element.
Any addition of the number of electrons to the neutral atom will make it negatively charged. Similarly any subtraction of the electrons will make it positively charged. Such an atom is known as ion and the process of charging the atom is termed an ionisation.
v. “The nuclear power engineering” is specially connected with variation of nucleons in nucleus. Protons and neutrons are the particles having the mass of about 1837 times and 1839 times the mass of an electron.
vi. The modern atomic theory tells that the atom has a diameter of about 10 mm. In a neutral atom the electrons are bound to the nucleus by the electrostatic forces, which follows the Coloumb’s law of forces, i.e., like charges repel and unlike charges attract each other. The function of electrostatic force is similar to the gravitational force.
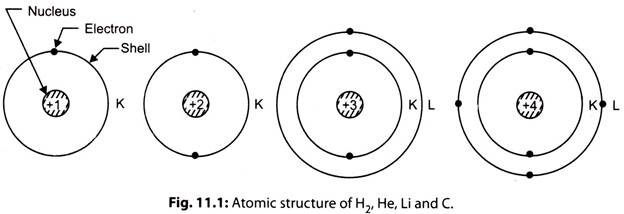
vii. The atomic spectrum study has revealed that every electron in an atom is in one group of specific states of motion which is corresponding to its total energy. In an atom the electrons are spinning around the nucleus in orbits. These orbits are called shells, which represent the energy levels for the electrons. All the electrons having very nearly the same total energy are said to be in the same shell.
The shells have been named as K, L, M, N etc. Each shell consists of the specific maximum number of electrons. The K shell (inner shell) contains 2 electrons, L shell has 8 electrons, M shell is limited to 18 and the N shell possesses 32 electrons. In fact, the number of electrons in any orbit is equal to In1 where n is the serial number of the orbit taking first orbit nearest to the nucleus, with the exception that the outermost orbit cannot have more than eight electrons.
In a given atom all orbits may not be complete. It is obvious from the study that amplitude difference in energy between two shells is much more than the difference in between energy levels in one shell. In a shell less than the specified number of electrons may exist but not a large number. The inner shell is filled up first and then the other successive shell are completed.
viii. The chemical properties of the atom varies with composition of number of electrons in various shells and the state of energies within the shells determine the electrical characteristics of the atom. For example, Hydrogen (H2) consists of one electron in the first shell, Helium (He) has two electrons in the first shell, Lithium (Li) has two electrons in first shell and one is second shell, Carbon (C) consists of two electrons in first and four in second shell.
ix. The electrons lying in the outermost shell are termed valence electrons. If the outermost shell is completely filled, the atom is stable and will not take any electron to fill up the gap. However, the incomplete outer shell will try to snatch the required number of electrons from the adjacent atom in a matter. The binding force between the electron and nucleus is the electrostatic force of attraction.
To emit one electron, energy required is more than the electrostatic force of attraction. When the energy is supplied, the electron jumps from one discrete energy level to another permissible level. The process starts from outer shell.
The electron possesses the energy in two forms, i.e., kinetic energy due to its motion and potential energy due to its position with respect to the nucleus. It is obvious that electrons cannot exist in between the permissible orbits.
x. The charge of nucleus is represented by the number of protons present. This number is known as atomic number and designated by the letter Z. It also shows the position of atom in the periodic table. ‘Hydrogen’ has only one number but natural ‘uranium’ has ninety two.
The atoms having higher atomic number have been developed artificially ranging from 93 to 102. These are einsteinium (Z = 99), Ferinium (Z = 100), and mendelevium (Z = 101). Platonium (Z = 94) is an important element to the nuclear power field.
The mass number (A) is the sum of total number of protons and neutrons in a nucleus. The number of electrons is represented by the letter N, i.e., N = (A – Z).
2. Essay on Atomic Mass Unit:
The mass of the atom is expressed in terms of the mass of the electron. The unit of mass has been considered as 1/16th of the mass of neutral oxygen atom which contains 8 protons and 8 neutrons. The atomic mass unit (a.m.u.) is equal to 1/16th the mass of oxygen neutral atom.
One a.m.u. = 1.66 x 10-24 g
It has been concluded that the density of matter in a nucleus is enormous. It has been investigated that the radius of nucleus is equal to 1.57 x 10-3 x 3√3, where A is the number of nucleons in nucleus.
The density of uranium by calculations comes to 1.65 x 1014 g/cm3. It has been found by calculations that natural substance has density millions of times lower than that of nuclear matter.
Electron Volt:
The energy is expressed in electron volt unit. An electron volt is equal to work done in moving an electron by a potential different of one volt. Or it is the amount of energy acquired by any particle with one electronic charge, when it falls through a potential of one volt.
One electron volt = 1.602 x 1019 joule.
3. Essay on Isotopes:
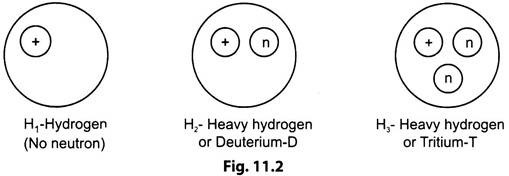
In any atom, the number of electrons is equal to number of protons. This is independent of neutrons in the nucleus. Atoms having different number of neutrons than the number of protons are known as ‘Isotopes’.
Examples:
Isotopes of hydrogen are shown in Fig. 11.2.
These isotopes have the same chemical properties and have the same atomic number and occupy the same place in the periodic table. But the nuclear properties of each of the isotopes are different because of the different number of neutrons in the nucleus.
The isotopes of oxygen vary from O14 to O19. The change of number of neutrons in nucleus affect the mass of atom.
Example:
Weight of heavy hydrogen is twice the weight of simple hydrogen. This means a volume of H2O weighs less than the same volume of D2O.
The isotopes can be represented with mass number (A) as subscript and atomic number (Z) as subscript like ZHA.
Example:
Hydrogen isotopes are represented as 1H (Deuterium), 1H3 (Tritium) and Uranium isotopes as U234, U235, U238.
The isotopes are not stable and disintegrate at a certain rate. The isotope which disintegrates at a fixed rate is called Radioactive isotope or Radio isotope.
The instability of the nucleus can be either by the separation of parent nucleus into 2 or more nuclei or by the rearrangement of nucleons in the matter so that there is an emission of particles or energy in the form of rays or by rearrangement of electrons. During this transformation there is emission of particles at a very high velocity. This is known as “radiation”.
We must note that for any specific isotope, the rate of radiation from a unit mass and also the energy distribution are fixed and cannot be changed by any method. Thus, for any isotope, the quantity of radiation per unit time can be determined easily.
4. Essay on Radioactivity:
Radioactivity was originally discovered by Becquerel in 1896. This phenomenon is confined almost entirely to the heaviest element from 83 to 106 in the periodic table.
The phenomenon of spontaneous emission of powerful radiations exhibited by heavy elements is called radioactivity. Radioactivity is essentially a nuclear phenomenon and is a drastic process because the element changes its kind. It is spontaneous and an irreversible self- disintegrating activity because the element breaks itself up for good. Those elements which exhibit this activity are called radioactive elements.
Examples are:
i. Uranium,
ii. Polonium,
iii. Radium,
iv. Radon,
v. Ionium,
vi. Thorium,
vii. Actinium,
viii. Mesothorium.
The radioactive radiations emitted by the radioactive elements are found to consist of the following:
(i) Alpha (α) rays or a-particles.
(ii) β rays or β-particles.
(iii) γ-rays or photons.
The radioactivity may be natural or artificial.
a. Natural Radioactivity:
It is that which is exhibited by element as found in Nature. It is always found in heavier elements in the periodic table.
b. Artificial or Induced Radioactivity:
The modern techniques of artificial transmutation of elements have made it possible to produce radioactivity in many other elements much lighter than those that occur in Nature. Such type of radioactivity is known as artificial or induced radioactivity.
The general properties of radioactive radiations are:
1. These radiations are highly penetrating, they affect photographic plates, ionise gases, cause scintillations on fluorescent screen, develop heat and produce chemical changes.
2. As radiations are given out, new elements are formed in an irreversible process— the new elements themselves being usually radioactive.
3. To emission of radiations is spontaneous and is not affected by external agents.
4. The emission is not instantaneous but is prolonged i.e., it is extended over a period of time otherwise it would not have been discovered at all.
5. Except for radioactivity, there is nothing abnormal about the radioactivity elements as regards their physical and chemical properties.
5. Essay on Radioactive Decay of an Element:
It has been observed that the emission of the particles in the form of alpha, beta or gamma radiations is not an instantaneous process. For various elements the decay time is different, which follows a certain law. Obviously the process is independent of the physical and chemical properties of the given isotope at a particular temperature and pressure.
The law states- “The small amount of disintegration of the isotope in a small period is directly proportional to the total number of radioactive nuclei and proportionality constant”.
If, N = Number of radioactive nuclei present at any time t,
N0 = Initial number of such nuclei,
λ = Proportionality constant (also known as disintegration constant or the radioactive decay constant of the material),
Then, the above law can be stated in the form of equation as follows:
The negative sign represents that during disintegration the number of the nuclei is decreasing.
Integrating the above equation (11.2) after proper arrangement within the proper limits, we get:
The eqn. (11.5) represents that the decay scheme follows the exponential law.
Activity:
The intensity of emitted radiation is termed activity.
This is directly dependent on the rate of disintegration of the element.
If, A = Activity at time t,
A1 = Initial activity,
k = Detection coefficient,
Half-Life:
Half-life represents the rate of decay of the radioactive isotopes. The half-life is the time required for half of the parent nuclei to decay or to disintegrate.
Here t1/2 is the half-life of radioactive nuclei. After passing every half-life the number of nuclei is reduced to half and so is the activity. This process is repeated for the several half-lives till the activity becomes negligible. The variation of half-life is from fraction of seconds to millions of years.
Half-life of some of the metals is given below:
Average (Mean) Life:
This indicates the average of total time for which the radioactive nuclei has disintegrated for several half-lives. Hence this is greater than half-life. This is obtained by taking the sum of the decay time of the radioactive nuclei and then it is divided by the initial number of nuclei.
If T is the time of average life, then
From the above eqns. it is clear that mean life is 1.445 times greater than half-life.
Note- Number of disintegrations per second is the unit of radioactivity and is termed curie, as this phenomenon was first discovered by Curie.
6. Essay on Nuclear Reactions within an Atom:
During a nuclear reaction, the change in the mass of the particle represents the release or an absorption of energy. If the total mass of the particle after the reaction is reduced, the process releases the energy, consequently, the increase in the mass of the resultant particle, will cause the absorption of energy.
The equations of nuclear reactions are connected with the resettlement of protons and neutrons within the atom. The equations are much similar to chemical reactions. The energy variation is also of the order of MeV. In simple term the equation shows the balance of neutron and proton.
A nuclear reaction is written as follows:
(i) The bombarded nuclei or the target nuclei is written first from left hand side.
(ii) In the middle within brackets, first is the incident particle and second one the ejected.
(iii) On the right hand side, the resultant nucleus is placed.
A neutron is written as:
0n1 because it has unit mass and it does not have any charge.
Any electron is written as- because its mass is negligible as compared to proton or neutron and its charge is equal but opposite to the charge of proton.
Some of the examples of reactions are given below:
(i) When 11Na23 is bombarded with protons possessing high energy, it is converted to 12Mg23
(where q = release or absorption of energy in the reaction)
(ii) When 13A127 is bombarded with high energy protons it is transformed to 14Si27.
(iii) When 13A127 is bombarded with deutrons, A128 and proton may be produced.
The eqns. (11.10), (11.11) and (11.12) may be written in the equation form as given below:
7. Essay on Fertile Materials:
It has been found that some materials are not fissionable by themselves but they can be converted to the fissionable materials, these are known as fertile materials.
Pu239 and U233 are not found in Nature but U238 and Th232 can produce them by nuclear reactions. When U238 is bombarded with slow neutrons it produces 92U239 with half-life of 23.5 days which is unstable and undergoes two beta disintegration. The resultant Pu239 has half-life of 2.44 x 104 yrs and is a good alpha emitter.
During conversion the above noted reactions will take place. The other isotopes of neptunium such as 2.1 day Np238 and plutonium can also be produced by the bombardment of heavy particles accelerated by the cyclotron.
The nuclear transformations to convert 90Th232 to U233 are given below:
U235 is the source of neutrons required to derive Pu239 and U233 from Th232 and U238 respectively. This process of conversion is performed in the breeder reactors.
Other Fissionable Materials:
Th227, Pa232, U231, Np238 and Pu241 are the other nuclides which are having high cross-sections for neutron thermal fission. Pu241 is the important nuclide which is used in plutonium fueled power reactors.
8. Essay on the Fission and Fusion of Neutrons:
Fission is the process that occurs when a neutron collides with the nucleus of certain of the heavy atoms, causing the original nucleus to split into two or more unequal fragments which carry off most of the energy of fission as kinetic energy. This process is accompanied by the emission of neutron and gamma rays.
The Chain Reaction:
A chain reaction is that process in which the number of neutrons keeps on multiplying rapidly (in geometrical progression) during fission till whole of the fissionable material is disintegrated. The chain reaction will become self-sustaining or self-propagating only if, for every neutron absorbed, at least one fission neutron becomes available for causing fission of another nucleus.
This condition can be conveniently expressed in the form of multiplication factor or reproduction factor (K) of the system which may be desired as:
If K > 1, chain reaction will continue and if K < 1, chain reaction cannot be maintained.
Nuclear fusion is the process of combining or fusing two lighter nuclei into a stable and heavier nuclide. In this case also, large amount of energy is released because mass of the product nucleus is less than the masses of the two nuclei which are fused.
Several reactions between nuclei of low mass numbers have been brought about by accelerating one or the other nucleus in a suitable manner. These are often fusion processes accompanied by release of energy.
However, reactions involving artificially- accelerated particles cannot be regarded as of much significance for the utilisation of nuclear energy. To have practical value, fusion reactions must occur in such a manner as to make them self-sustaining, i.e., more energy must be released than is consumed in initiating the reaction.
It is thought the energy liberated in the sun and other star of the main sequence type is due to the nuclear fusion reactions occurring at the very high stellar temperature of 30 million °K. Such processes are called thermonuclear reactions because they are temperature- dependent.