Are you looking for an essay on ‘DNA Replication and Structure’? Find paragraphs, long and short essays on ‘DNA Replication and Structure’ especially written for college and medical students.
Essay on DNA Replication and Structure
Essay Contents:
- Essay on the Introduction to DNA Replication
- Essay on Meselson and Stahl Experiment
- Essay on the Machine for DNA Replication: The Replisome
- Essay on Features of DNA Replication
- Essay on the Types of DNA Replication
- Essay on Replicating the Ends of Chromosomes—Telomeres and Telomerase
- Essay on the Post-Replicative Modification of DNA: Methylation
Essay # 1. Introduction to DNA Replication:
Chromosomes are structures within a cell which contain the genetic information that is passed on from one generation to the next. Specific regions, called genes, on each chromosome contain the hereditary information which distinguishes individuals from each other. The genes also contain the coded information required for the synthesis of proteins and enzymes needed for the normal functions of the cells. Bacterial cells may have 1000 genes, while the human cell contains more than a million genes.
In prokaryotes, there is usually a large circular chromosome. In addition, there may be several smaller circular chromosomes called plasmids. None of these is enclosed in a membrane; they merely drift around within the cytoplasm of the prokaryote cell. A single E. coli (bacteria) chromosome of double helical DNA consists of 3.4 million base pairs. In eukaryotes, most chromosomes are located in the cell nucleus and are composed of protein and DNA. Smaller chromosomes exist in mitochondria and chloroplasts – these tend to regulate activity solely within the organelle in which they occur.
DNA replication is the basis for biological inheritance. Prior to cell division, the DNA material in the original cell must be duplicated so that after cell division, each new cell contains the full amount of DNA material. The process of DNA duplication is usually called replication. The replication is termed semiconservative since each new cell contains one strand of original DNA and one newly synthesized strand of DNA.
The original polynucleotide strand of DNA serves as a template to guide the synthesis of the new complementary polynucleotide of DNA. Cellular proof-reading and error-checking mechanisms ensure nearly perfect fidelity of the DNA copies. DNA replication commences at specific locations in the genome called “origins”. The DNA unwinds at the origin to form a replication fork. When cells prepare to divide, either for growth or for repair, the chromosomes become visible as duplicated threads, the chromatids. For chromatids to form, the DNA of the original chromosomes needs to be reproduced so that each new cell will ultimately possess the correct genetic information to function appropriately.
DNA replication occurs when the complementary strands of DNA break apart and unwind. This is accomplished with the help of enzymes called helicases. Additional enzymes and proteins attach to the individual strands, holding them apart and preventing them from coiling upon them. The point at which the double helix separates is called the replication fork, because of the shape of the molecule.
At this site enzymes called DNA polymerases move along each of the separated DNA strands, adding nucleotides to the exposed bases according to the base pairing rules. The ribose-phosphate bonds form between the new nucleotides to hold the new strand together. The process continues until the original double helix is completely unwound and two new double helices have been formed. Each new double helix is composed of one old DNA strand and one new strand. This is described as semi- conservative replication.
For heredity, biological information must be accurately copied and transmitted from each cell to all of its progeny.
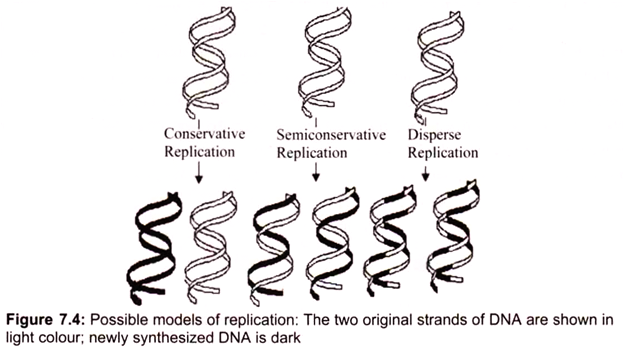
Three ways for DNA molecules to replicate may be considered, each obeying the rules of complementary base pairing (Fig.7.4):
i. Conservative replication would leave intact the original DNA molecule and generate a completely new molecule.
ii. Dispersive replication would produce two DNA molecules with sections of both old and new DNA interspersed along each strand.
iii. Semiconservative replication would produce molecules with both old and new DNA, but each molecule would be composed of one old strand and one new one. The replication is semiconservative. Each strand acts as a template for the synthesis of a new DNA molecule by the sequential addition of complementary base pairs, thereby generating a new DNA strand that is the complementary sequence to the parental DNA. Each daughter DNA molecule ends up with one of the original strands and one newly synthesized strand (Fig. 7.4).
Essay # 2. Meselson and Stahl Experiment:
The Meselson-Stahl experiment was an important step in developmental biology research. Two scientists, Matthew Meselson and Franklin Stahl carried out their experiment in 1958 to prove the semiconservative DNA replication hypothesis proposed by James Watson and Francis Crick.
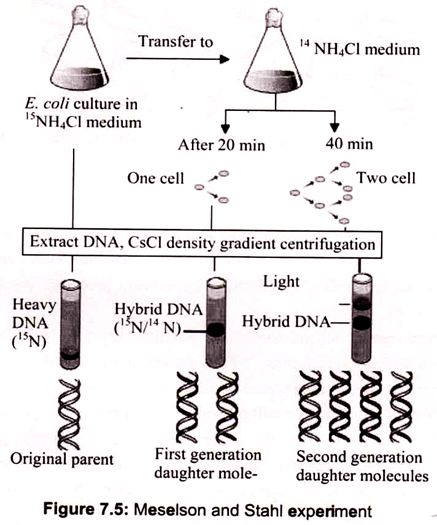
The experiment carried out by Meselson and Stahl involved growing a culture of Escherichia coli in a medium containing 15NH4Cl (ammonium chloride labeled with the heavy isotope of nitrogen). Cells were then transferred to normal medium (containing 14NH4Cl) and samples taken after 20 minutes (one cell division) and 40 minutes (two cell divisions). DNA was extracted from each sample and the molecules analyzed by density gradient centrifugation. After 20 minutes the entire DNA contained similar amounts of 14N and 15N, but after 40 minutes two bands were seen, one corresponding to hybrid 14N-15N-DNA, and the other to DNA molecules made entirely of 14N.
The banding pattern seen after 20 minutes enables conservative replication to be discounted because this scheme predicts that after one round of replication there will be two different types of double helix, one containing just 15N and the other containing just 14N. The single 14N-15N-DNA band that was actually seen after 20 minutes is compatible with both dispersive and semiconservative replication, but the two bands seen after 40 minutes are consistent only with semiconservative replication.
Dispersive replication continues to give hybrid 14N-15N molecules after two rounds of replication, whereas the granddaughter molecules produced at this stage by semiconservative replication include two that are made entirely of 14N-DNA. Now the density gradient revealed two bands of DNA, the first corresponding to a hybrid composed of equal parts of newly synthesized and old DNA, and the second corresponding to molecules made up entirely of new DNA (Fig. 7.5).
This result agrees with the semi-conservative scheme but is incompatible with dispersive replication; the latter predicting that after two rounds of replication all molecules would be hybrids. The second result, after two generations, showed that one part of the DNA had intermediate density and the other part had light density. This ruled out dispersive replication as in that case the DNA distribution would have been same between the strands and the resulting density would have been lower than the intermediate one.
Essay # 3. Machine for DNA Replication: The Replisome:
The replisome is a complex molecular machine that carries out replication of DNA. It is comprised of a number of subcomponents, each performing a specific function during the process of replication.
(a) Helicase:
Helicase is an enzyme which breaks the hydrogen bonds between the two strands of DNA, thus separating the strands ahead of DNA synthesis. As helicase unwinds the double helix, it induces the formation of supercoils in other areas of the DNA.
(b) Gyrase:
Gyrase relaxes and removes the supercoiling which has been caused by the helicase by cutting the DNA strands, allowing it to rotate and release the supercoil, and then rejoining the strands. Gyrase is most commonly located upstream of the replication fork – where the supercoils are being formed.
(c) Primase:
Primase adds complementary RNA primers to a DNA strand to begin Okazaki fragments as well as leading strand since DNA polymerase can only continue (but not begin) a strand.
(d) DNA Polymerases:
All DNA polymerases, whether from prokaryotic or eukaryotic sources, share the following properties:
i. The incoming base is selected within the DNA polymerase active site, as determined by Watson-Crick geometric interactions with its corresponding base in the template strand,
ii. chain growth is in the 5′ —> 3′ direction and is anti- parallel to the template strand, and
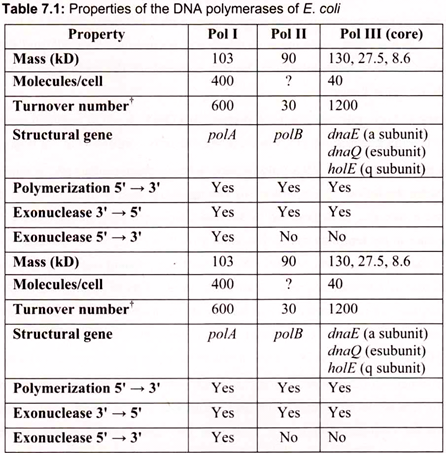
iii. DNA polymerases cannot initiate DNA synthesis de novo—all require a primer oligonucleotide with a free 3-OH to build upon. DNA polymerase enzymes are numbered I, II, and III, in order of their discovery. DNA polymerases I and II function principally in DNA repair; DNA polymerase III is the chief DNA-replicating enzyme of E. coli. Only 10 to 20 copies of this enzyme are present per cell (Table 7.1).
One mechanism intrinsic to virtually all DNA polymerases is a separate 3’—> 5′ exonuclease activity that serves to double-check each nucleotide after it is added. This nuclease activity permits the enzyme to remove a nucleotide just added and is highly specific for mismatched base pairs. If the wrong nucleotide has been added, translocation of the polymerase to the position where the next nucleotide is to be added is inhibited. The 3’—>5′ exonuclease activity removes the mispaired nucleotide, and the polymerase begins again. This activity, called proofreading, is not simply the reverse of the polymerization reaction, because pyrophosphate is not involved.
The mechanics of DNA replication was originally characterized in the bacterium, E. coli which contains 3 distinct enzymes capable of catalyzing the replication of DNA. These have been identified as DNA polymerase (pol) I, II, and III. Pol I is the most abundant replicating activity in E. coli but has as its primary role to ensure the fidelity of replication through the repair of damaged and mismatched DNA. Replication of the E. coli genome is the job of pol III.
This enzyme is much less abundant than pol I, however, its activity is nearly 100 times that of pol I. The DNA polymerase I is used to fill the gap between DNA fragments of the lagging strand. It is also the major enzyme for gap filling during DNA repair. The DNA polymerase II is encoded by the PolB gene, which is involved in the SOS response to DNA damage. DNA replication is mainly carried out by the DNA pol III.
(i) DNA Polymerase I:
In 1957, Arthur Romberg and his colleagues discovered the first DNA polymerase, DNA polymerase I, awarded the Nobel Prize in physiology in 1959. DNA polymerase I catalyzed the synthesis of DNA in vitro, if provided with all four deoxynucleo- side-5′-triphosphates (dATP, dTTP, dCTP, dGTP), a template DNA strand to copy, and a primer. A primer is essential because DNA polymerases can elongate only pre-existing chains; they cannot join two deoxyribonucleoside-5’-phosphates together to make the initial phosphodiester bond.
The primer base-pairs with the template DNA, forming a short, double -stranded region. This primer must possess a free 3′-OH end to which an incoming deoxynu- cleoside monophosphate is added. All four dNTPs are substrates to polymerase, while pyrophosphate (PPi) is released following the formation of the bond, and the dNMP is linked to the 3-OH of the primer chain through formation of a phosphodiester bond. The deoxynu- cleoside monophosphate to be incorporated is chosen through its geometric fit with the template base to form a Watson-Crick base pair.
The reaction can be written as:
(DNA)n + dNTP <—> (DNA)n+1 + PPi
As DNA polymerase I catalyzes the successive addition of deoxynucleotide units to the 3′-end of the primer, the chain is elongated in the 5′ —> 3′ direction, forming a polynucleotide sequence that runs antiparallel to the template but complementary to it. DNA polymerase I can precede along the template strand, synthesizing a complementary strand of about 20 bases before it dissociates from the template. The degree to which the enzyme remains associated with the template through successive cycles of nucleotide addition is referred to as its processivity.
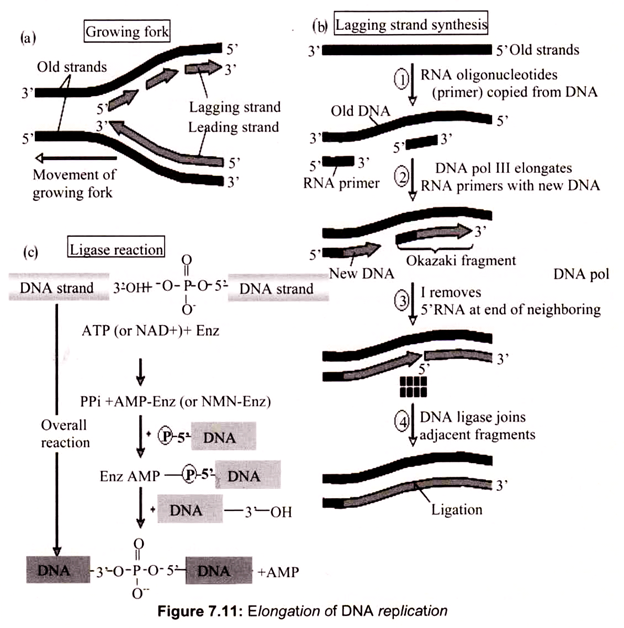
DNA polymerase I remove the RNA primers set by primase and completes the Okazaki fragments. As there is such a small gap remaining after the action of DNA polymerase I have continued the strand of the Okazaki fragment, ligases is required to fill in the gap. The two ends of the Okazaki fragments are subsequently connected by covalent bonds. Single-strand binding proteins bind to the exposed bases in.an effort to counteract their instability and prevent the single-strand DNA from hydrogen-bonding to itself to form dangerous hairpin structures. DNA polymerases contain a ‘proofreading’ mechanism, commonly referred to as ‘exonuclease activity’. This removes nucleotides that have been mistakenly added.
E. coli DNA polymerase I is a 109-kD protein consisting of a single polypeptide of 928 amino acid residues. In addition to its 5′ —> 3′ polymerase activity, DNA polymerase I has two other catalytic functions, a 3′ —> 5′ exonuclease (3′-exonuclease) activity and a 5′ —> 3′ exonuclease (5’-exonuclease) activity. The three distinct catalytic activities of DNA polymerase I reside in separate active sites in the enzyme. As shown by Hans Klenow, the DNA polymerase I polypeptide chain can be cleaved into two fragments by limited proteolysis with subtilisin or trypsin. The smaller fragment (residues 1 through 323) contains the 5’ – exonuclease activity, whereas the larger fragment (residues 324 through 928, the so-called Klenow fragment) has the polymerase and3-exonuclease activities.
The exonuclease activities of E. coli DNA polymerase I serve proofreading and editing functions that enhance the accuracy of DNA replication. The 3′-exonuclease activity removes nucleotides from the 3′-end of the growing chain an action that apparently negates the effects of the polymerase activity. Its purpose, however, is to remove incorrect (mismatched) bases.
(ii) DNA Polymerase II:
The enzyme is 90 kDa in size and is coded by the polB gene. It has 3-5′ exonuclease activity and involved in repair of damaged DNA. It is unable to replicate long single strands with short complementary primer. Strains of E. coli with mutations in polymerase II were found to grow and otherwise behave normally, so the role of this enzyme in the cell is unknown. Pol II has a low error rate but it is too much slow to be of any use in normal DNA synthesis. Pol II differs from Pol I in that it lacks a 5’—>3′ exonuclease activity, and cannot use a nicked duplex template.
(iii) DNA Polymerase III:
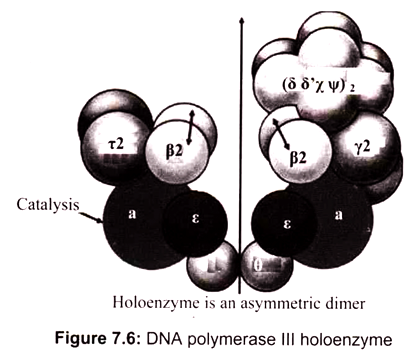
DNA polymerase III is comprised of two catalytic cores-one for replication of the leading strand and one for the lagging strand. DNA polymerase III, however, cannot stay on the DNA strand long enough to efficiently replicate a daughter strand. Hence, DNA polymerase III stays on the strands via a dimer beta clamp which contains three subunits that come together to enclose the strand. It is the main polymerase in bacteria (elongates in DNA replication); has 3’—> 5′ exonuclease proofreading ability. It is 15 times more active than DNA Pol I and 300 times than DNA Pol II. DNA Polymerase III (pol III) from E. coli is a single protein of molecular weight 130 kDa (130,000 grams per mole). It is also referred to as polC, dnaE, or the alpha subunit. DNA pol III works to replicate DNA in the bacterial cell in conjunction with other proteins. This multi-protein complex is referred to as the pol III holoenzyme.
The DNA polymerase III consists of several subunits, among them α, ԑ, and θ subunits constitute the core polymerase. The major role of other subunits is to keep the enzyme from falling off the template strand. Two β subunits can form a donut-shaped structure to clamp a DNA molecule in its center, and slide with the core polymerase along the DNA molecule. By acting as a sliding clamp, beta helps the holoenzyme to replicate long stretches of DNA without falling off the strand. Pol III holoenzyme directs both leading and lagging strand synthesis simultaneously by virtue of having two polymerase subunits (Fig. 7.6). This allows continuous polymerization of up to 5 x 105 nucleotides. In the absence of β-subunits, the core polymerase would fall off the template strand after synthesizing 10-50 nucleotides.
The α subunit carries out the catalytic polymerase function. The e subunit performs the 3′ —> 5′ exonuclease activity and contributes proofreading ability to the “core” polymerase. The role of subunit θ is unknown. The γ complex is responsible for assembly of the DNA polymerase III holoenzyme complex onto DNA. The γ complex of the holoenzyme acts as a clamp loader by catalyzing the ATP-dependent transfer of a pair of β- subunits to each strand of the DNA template. Epsilon functions as 3-to-5′ exonuclease (editing exonuclease). Theta subunit stimulates 3′-to-5′ exonuclease while Tau dimerizes cores and activates DnaB helicase activity. These are sub-assembled in core enzyme.
The beta subunit can be loaded onto DNA by the clamp loader (gamma complex) in an ATP-dependent reaction. Beta cannot be loaded onto linear DNA , covalently closed circular DNA, or single-stranded circular DNA, but it can be loaded onto nicked circles, gapped circles, and primed single-stranded circles; that is, clamp loader requires and recognizes a 3′- hydroxyl-terminus (primer-terminus).
Once loaded onto a nicked circle, beta stays associated with the DNA. However, linearization of the nicked circle with a restriction endonuclease releases beta from the DNA; that is, beta is a sliding clamp. It can slide along double- stranded DNA (or DNA-RNA in double-stranded form), but cannot slide on single-stranded DNA or single-stranded DNA coated with SSB.
(e) Eukaryotic DNA Polymerase:
Eukaryotic cells contain five DNA polymerases- α, β, γ, δ, and ε. Polymerase y is located in mitochondria and is responsible for replication of mitochondrial DNA. The other four enzymes are located in the nucleus and are therefore candidates for involvement in nuclear DNA replication. Polymerases α, δ, and ε are most active in dividing cells, suggesting that they function in replication. In contrast, polymerase β is active in non-dividing and dividing cells, indicating that it may function primarily in the repair of DNA damage.
Two types of experiments have provided evidence to the roles of polymerases α, δ, and ε in DNA replication:
First, replication of the DNAs of some animal viruses, such as SV40, can be studied in cell-free extracts. The ability to study replication in vitro has allowed direct identification of the enzymes involved, and analysis of such cell-free systems has shown that polymerases α and δ are required for SV40 DNA replication.
Second, polymerases α, δ, and ε are found in yeasts as well as in mammalian cells, enabling the use of the powerful approaches of yeast genetics to test their biological roles directly. Such studies indicate that yeast mutants lacking any of these three DNA polymerases are unable to proliferate, implying a critical role for polymerase e as well as for α and δ. However, further studies have shown that the essential function of polymerase s in yeast does not require its activity as a replicative DNA polymerase.
(f) DNA Ligase:
DNA ligase forms new bonds between adjacent nucleotides by forming phosphodiester bond, cooperates with DNA polymerase, repairs single strand nicks in the duplex DNA, joins fragments of DNA in the process of recombination which occurs during genetic transformation and transduction in bacteria.
Essay # 4. Features of DNA Replication:
a. Replication is Bidirectional:
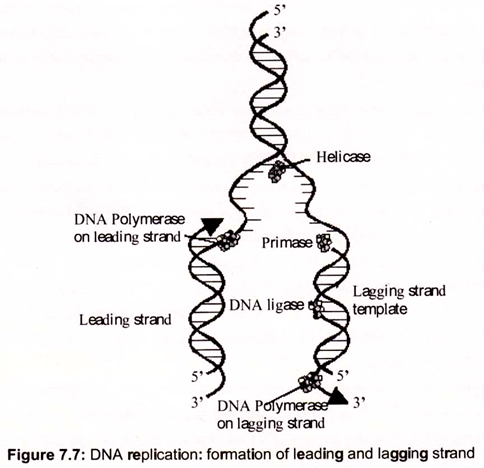
Replication of DNA molecules begins at one or more unique sites called origin(s) of replication and, except in certain bacteriophage chromosomes and plasmids, proceeds in both directions from the origin. For example, replication of E. coli DNA begins at oriC, a unique 245-bp site. From this site, replication advances in both directions around the circular chromosome. That is, bidirectional replication involves two replication forks, which move in opposite directions by unwinding the DNA helix (Fig. 7.7).
Semiconservative replication depends on unwinding the DNA double helix to expose single- stranded templates to polymerase action. For a double helix to unwind, it must either rotate about its axis (while the end of its strands are held fixed), or positive supercoils must be introduced, one for each turn of the helix unwound. If the chromosome is circular, as in E. coli, only the latter alternative is available, because DNA replication in E. coli proceeds at a rate approaching 1000 nucleotides per second, and there are about 10 bp per helical turn, the chromosome would accumulate 100 positive supercoils per second, as result the DNA would become too tightly supercoiled to allow unwinding of the strands.
DNA gyrase, a Type II topoisomerase, acts to overcome the torsional stress imposed upon unwinding by introducing negative supercoils at the expense of ATP hydrolysis. The unwinding reaction is driven by helicases, a class of proteins that catalyze the ATP- dependent unwinding of DNA double helices. Unlike topoisomerases that alter the linking number of dsDNA through phosphodiester bond breakage and reunion, helicases simply disrupt the hydrogen bonds that hold the two strands of duplex DNA together. A helicase molecule requires a single-stranded region for binding. It then moves along the DNA strand, its translocation coupled to ATP hydrolysis and to strand unwinding. SSB (ssDNA-binding protein), binds to the unwound strands, preventing their re-annealing. At least 10 distinct DNA helicases involved in different aspects of DNA and RNA metabolism have been found in E. coli alone.
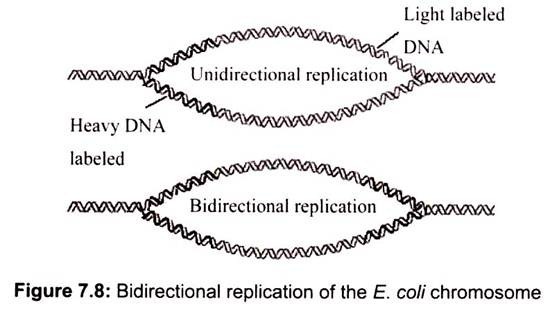
Unidirectional versus bidirectional, replication can be resolved if cells grown several generations in the presence of low amounts of radioactive 3H-thymidine to lightly label the chromosome. They are then exposed briefly to high amount of 3H-thymidine to cause heavy labeling in newly synthesized regions of DNA. If replication is unidirectional, only one advancing replication fork is present and only the DNA adjacent to it should be heavily labeled. If replication is bidirectional, autoradiograms of replicating chromosomes should show two replication forks heavily labeled with radioactive thymidine (Fig. 7.8).
b. Replication is Semi-Discontinuous:
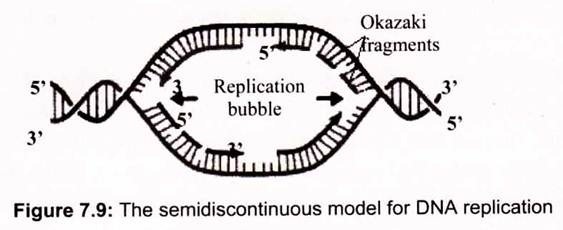
The autoradiographic results indicate that the two strands of duplex DNA are both replicated at each advancing replication fork by DNA polymerase. DNA polymerase uses ssDNA as a template and makes a complementary strand by polymerizing deoxynucleotides in the order specified by their base-pairing with bases in the template. DNA polymerases synthesize DNA only in a 5′ —> 3′ direction, reading the antiparallel template strand in a 3′ —> 5′ sense. A dilemma arises- How does DNA polymerase copy the parent strand that runs in the 5′ —> 3’direction at the replication fork? It turns out that the two daughter strands are synthesized in different ways so that replication is semi-discontinuous (Fig. 7.9).
As the DNA helix is unwound during its replication, the 3′ —> 5′ strand (as defined by the direction that the replication fork is moving) can be copied continuously by DNA polymerase proceeding in the 5′ —> 3′ direction behind the replication fork. The other parental strand is copied only when a sufficient stretch of its sequence has been exposed for DNA polymerase to move along it in the 5′ —> 3′ mode. Thus, one parental strand is copied continuously to give a newly synthesized copy, the leading strand; the other parental strand is copied in an intermittent, or discontinuous, mode to yield a set of fragments. These fragments are then joined to form an intact lagging strand.
c. The Lagging Strand is Formed from Okazaki Fragments:
The leading strand is synthesized as one continuous polynucleotide, beginning at the origin and ending at the termination site. In contrast, the lagging strand is synthesized discontinuously in short pieces in the direction opposite fork movement. These pieces of lagging strand are then joined by a separate reaction.
In 1968, Tuneko and Reiji Okazaki provided biochemical verification of the semi-discontinuous pattern of DNA replication described above. The Okazakis exposed a rapidly dividing E. coli culture to 3H-labeled thymidine for 30 seconds, quickly collected the cells, and found that half of the label incorporated into nucleic acid appeared in short ssDNA chains just 1000 to 2000 nucleotides in length. (The residual radioactivity was recovered in very large DNA molecules). Subsequent experiments demonstrated that, with time, the newly synthesized short ssDNA Okazaki fragments became covalently joined to form longer polynucleotide chains, in accord with a semi-discontinuous mode of replication. This semi-discontinuous mode of replication has been established with the help of electron micrographs of DNA undergoing replication in eukaryotic cells.
d. Speed of Replication:
The single molecule of DNA that is the E. coli genome contains 4.7 x 106 nucleotide pairs. DNA replication begins at a single, fixed location in this molecule, the replication origin, proceeds at about 1000 nucleotides per second, and thus is done in no more than 40 minutes.
The average human chromosome contains 150 x 106 nucleotide pairs which are copied at about 50 base pairs per second. The process would take a month (rather than the hour it actually does) but for the fact that there are many places on the eukaryotic chromosome where replication can begin. Replication begins at some replication origins earlier in S phase than at others, but the process is completed for all by the end of S phase. As replication nears completion, “bubbles” of newly replicated DNA meet and fuse, finally forming two new molecules.
Essay # 5. Types of DNA Replication:
a. Prokaryotic DNA Replication:
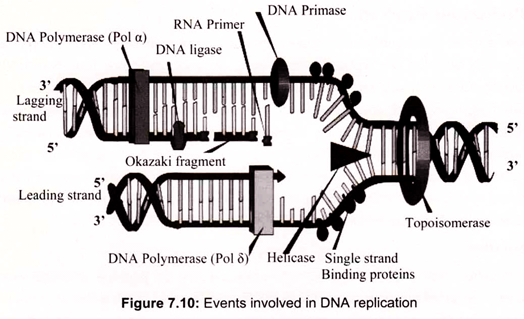
Replication of the prokaryotic chromosome begins at a single replication origin and proceeds bidirectionally until the two replication forks meet. At each replication fork, both leading and lagging strand synthesis are catalyzed by a single multi-protein replication machine, the so-called replisome, which consists of DNA-unwinding proteins; the priming apparatus, or primosome, which is needed to initiate, or prime DNA replication; and DNA polymerase III holoenzyme with two equivalents of “core” polymerase, one for the leading strand and one for the lagging strand. As this replisome follows the replication fork, the template for lagging strand synthesis (the strand running 5′ —> 3′ in the direction of fork movement) must be looped around so that it can be read in the 3′ —> 5′ direction (Fig. 7.10).
i. Initiation:
In the initiation step, origin of replication is unwound, and the partially unwound strands form a replication bubble, with one replication fork on either end. Each group of enzymes at the replication fork (pre-replication complex) moves away from the origin, unwinding and replicating the original DNA strands as they proceed. The pre-replication complex consists of helicase, single-strand binding proteins (SSB) and a primase, which generates an RNA primer to be used in DNA replication.
Replication of the E. coli chromosome is initiated at a unique site, oriC. Within oriC are four 9-bp repeats. DnaA protein, a 52-kD polypeptide, is the initiation factor, which recognizes and binds to these repeats. DnaA protein binding is cooperative; once the four 9-bp repeats are occupied, 20 to 40 additional DnaA monomers bind so that the entire oriC region, is complexed with DnaA protein. HU, a histone-like protein, prevents nonspecific initiation at sites other than oriC. The resulting complex resembles a nucleosome, with negatively supercoiled oriC DNA wrapped around a DnaA core. The DnaA protein then mediates the separation of the strands of the DNA duplex by acting on three AT-rich tandem repeats located at the 5′-end of the sequence defining oriC.
Formation of this open complex by DnaA protein is ATP-dependent. Next, DnaB protein binds to the open oriC. DnaB protein is delivered to oriC by DnaC protein, but DnaC protein does not enter the protein assemblage at oriC. Delivery of DnaB protein by DnaC protein is assisted by DnaT protein. The addition of DnaB protein completes assembly of the pre- priming complex.
ATP hydrolysis drives the formation of this complex. DnaB protein has helicase activity and it further unwinds the DNA in the pre-priming complex in both directions, assisted by DNA gyrase. SSB tetramers coat single-stranded regions as they arise. Unwinding exposes the base sequence of the strands so that RNA primers can be synthesized by primase, and the strands can be read as templates by the replicative polymerase.
ii. Elongation:
After the helicase unwinds the DNA, single-strand binding protein is used to hold the DNA strands apart. RNA primase is then bound to the starting DNA site. At the beginning of replication, an enzyme called DNA polymerase binds to the RNA primase, which indicates the starting point for the replication. DNA polymerase can only synthesize new DNA from the 5′ —> 3′ (of the new DNA), because of this, the DNA polymerase can only travel on one side of the original strand without any interruption. This original strand, which goes from 3’—>5′, is called the leading strand.
The complement of the leading strand, from 5’—>3′ is the lagging strand. Each time the helicase unwinds additional DNA, a (potentially) new DNA polymerase needs to be added. As a result, the DNA of the lagging strand is replicated in a piecemeal fashion. Another enzyme, DNA ligase, is used to connect the so-called Okazaki fragments. Coupled leading strand and lagging strand synthesis is achieved by the action of the pol III holoenzyme.
Two “core” DNA polymerase III units are present. One is synthesizing the leading strand, using the parental 3′ —> 5′ strand as template. The other synthesizes the lagging strand using the 5′ —> 3′ parental strand as template. The lagging strand template must be looped out so that it can be copied in the 3′ —> 5′ direction. The “core” pol III on this lagging strand has completed the synthesis of an RNA-primed Okazaki fragment when it encounters the 5′-end of the previous fragment. Synthesis of the next RNA primer by the primosome triggers the γ complex to disassemble the β2-sliding clamp, releasing the lagging strand from the DNA polymerase III holoenzyme. The γ complex assembles a new β2-sliding clamp at the 3-OH end of the next RNA primer, re-attaching the lagging strand “core” polymerase III to begin a new round of lagging strand synthesis (Fig. 7.11).
The double helix must be unwound ahead of the advancing replication fork. Unwinding is driven by ATP hydrolysis; DnaB protein has the helicase activity associated with the primosome. DnaB moves in the 5′ —> 3′ direction along the leading strand template, hydrolyzing 2 ATPs for each base pair that it separates. Protein-protein interaction between a t-subunit of the DNA polymerase III holoenzyme and DnaB is essential for rapid replication fork progression. PriA protein is, like DnaB, a helicase associated with the primosome, but PriA moves 3′ —> 5′ along the lagging strand. The binding of SSB protein to the single- stranded regions created behind these proteins prevents re-annealing.
iii. Termination:
Replication termination of prokaryotic and of some eukaryotic chromosomes occurs at specific sequences called replication termini. In Escherichia coli, there are 10 replication termini (Ter) located in a region diametrically opposite to the replication origin. The Ter sites have polarity, i.e., they arrest replication forks, when they are present in one orientation with respect to ori, but allow forks to pass through unimpeded in the opposite orientation. The Ter sites are located in two clusters of 5 each, with each cluster having a polarity opposite to that of the other. Thus, the arrangement of the Ter sites forms a replication trap that forces the two forks, initiated at oriC, to meet each other within a well-defined region of the chromosome
The Ter sites specifically interact with the replication terminator protein called Tus, which is a polar contrahelicase, i.e., it impedes the DNA unwinding activity of DnaB in an orientation-dependent manner. The crystal structure of the Tus—Ter complex has been solved, and it reveals a bilobed protein that has structural asymmetry and has a DNA-binding domain, consisting of a series of β-strands that invade the major groove of Ter DNA.
The crystal structure was originally interpreted to account for replication fork arrest, solely on the basis pf Tus-7er, protein-DNA interaction, that supposedly was strong enough to form a nonspecific barrier, not only to DnaB helicase-catalyzed DNA unwinding, but also, in principle, to any protein that would unwind DNA. Tus protein is a contrahelicase. That is, Tus protein prevents the DNA duplex from unwinding by blocking progression of the replication fork and inhibiting the ATP-dependent DnaB helicase activity.
b. Eukaryotic DNA Replication:
The fundamental enzymology of eukaryotic DNA replication has been elucidated from yeast genetic studies and biochemical studies on the replication of the simian virus 40 (SV40) genome in vitro. In addition to SV40 and yeast models, adenovirus and herpes simplex virus, of which the genetics are well established, have also been used to gain an understanding of the protein machinery that functions at the replication fork during duplication of cellular DNA in eukaryotes.
DNA replication requires the coordinated action of several proteins and enzymes to ensure efficient and accurate duplication of the genome. DNA replication is initiated from chromosomal sites termed origins, where two replication forks are established which proceed in opposite directions until the next replication unit is reached. At least two DNA polymerases, Pols α and δ function at the replication fork.
Processive DNA synthesis is guaranteed by their accessory proteins- proliferating cell nuclear antigen (PCNA), which forms a clamp structure around DNA, and a clamp-loader protein, replication factor C (RFC). DNA primase, single stranded DNA binding protein RPA (replication protein A), DNA topoisomerases, DNA helicases, RNase H, DNA ligases, and other factors are needed for efficient replication of the genome. The structure of these proteins appears to be conserved in all the eukaryotic organisms analyzed so far, and their function in DNA replication has been established by reconstitution experiments in vitro with purified proteins.
The mechanism of DNA replication in eukaryotic cells shows strong parallels with prokaryotic DNA replication, but the situation in eukaryotes is vastly more complex. For example, in a growing human cell, some 6 billion bp of DNA must be duplicated with high fidelity once in each cell cycle. The events associated with cell growth and division in eukaryotic cells fall into a general sequence having four distinct phases, M, G1, S, and G2 .Eukaryotic cells have solved the problem of replicating their enormous genomes in the few hours allotted to the S phase by initiating DNA replication at multiple origins of replication distributed along each chromosome.
Depending on the organism and cell type, replication origins, also called replicators, occur every 3 to 300 kbp (for example, an average human chromosome has several hundred replication origins). In lower eukaryotes such as yeast, replicators are small discrete chromosomal regions (100-200 bp), but in mammalian chromosomes, the zones where initiation of DNA replication occurs may span 500 to 50,000 bp. So that eukaryotic DNA replication can proceed concomitantly throughout the genome, each eukaryotic chromosome contains many units of replication, so-called replicons.
i. Initiation of Eukaryotic DNA Replication:
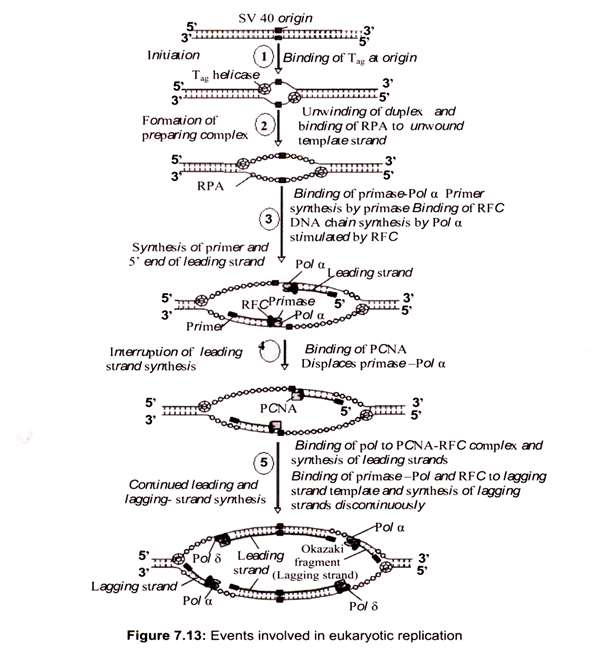
Saccharomyces cerevisiae is an excellent model system to study the initiation of chromosome replication, since the origins exist as short, well-defined sequences of about 150 bp. These origins, termed autonomously replicating sequences (ARSs). In mammalian cells the replication origin apparently consists of a large chromosomal region permissive for initiation, within which some regions are more frequently used than others.
DNA synthesis is initiated by the binding of initiator proteins to the origins of replication. In S. cerevisiae a multi-subunit protein called the origin recognition complex (ORC) binds specifically to ARS sequences. This complex consists of six proteins, Orcl p-Orc 6p, which are all essential for viability and initiation of DNA replication. In S. cerevisiae, the ORC complex is bound to origins in all stages of the cell cycle and forms the core of the origin complex to which other components are loaded in a step-wise manner.
The binding of Cdc6 protein to the ORC is essential for the next step in initiation, which is loading of MCM (mini chromosome maintenance) proteins onto origins. A complex of ORC, Cdc6 and MCMs forms a pre-replicative complex called the pre-RC, which is established at the end of mitosis, after separation of sister chromatids. Recently, a novel loading factor for MCM proteins, Cdtl, was identified in both S. pombe and Xenopus, which appears to function co-ordinately with Cdc6. At the onset of S-phase, the pre-RC has to be converted into an initiation complex that leads to initiation of DNA synthesis. This activation of pre- RC is accomplished by the action of S-phase specific cyclin-dependent kinases (CDKs) and Dbf4-dependent kinases (DDKs), which activate the firing of replication origins.
ii. Assembly of Replicative Complexes on to Origins:
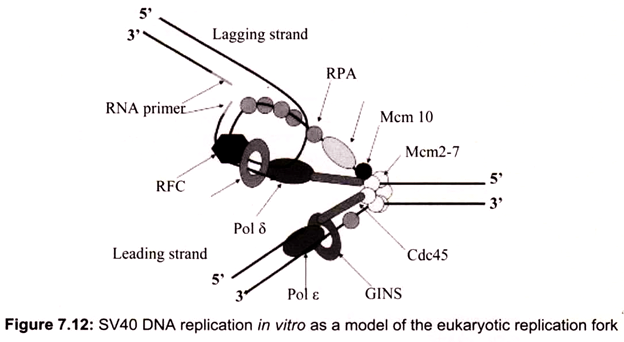
Initiation requires a stepwise association of proteins with replication origins before DNA synthesis can begin. The origin recognition complex (ORC) binds to DNA and provides a site on the chromosome where additional replication factors can associate (Fig. 7.12). An early step leading to initiation, called licensing or pre-replicative complex formation, involves the association of Mcm2-7 complex with DNA at ORC, in a process requiring Cdt1 and Cdc6. Mcm2-7 proteins provide helicase activity for DNA synthesis and loading of these proteins confers competence on the origin to fire in S phase.
Onset of DNA synthesis requires the action of two protein kinases (cyclin dependent kinase (CDK) and Cdc7), which trigger the association of additional proteins with the origin, such as Cdc45 and GINS (Go, Ichi, Nii, and San; five, one, two, and three in Japanese). During the process of initiation, DNA polymerases are also recruited and DNA synthesis starts. During replication, Mcm2-7 proteins move away from the origin and further assembly of pre-replicative complexes is blocked. This ensures that origins can only fire a single time per cell cycle.
In the conversion of the pre-RC complex into an initiation complex, Cdc6 and MCM proteins are released from the pre-RC complex and DNA polymerases are recruited onto origins to assemble a replicative complex. This assembly process takes place in a step-wise manner and the loading of Cdc45 is required for the loading of other replication proteins, such as the eukaryotic single-stranded DNA binding protein RPA. The ability of RPA to bind single-stranded DNA is essential for the first step of DNA replication, unwinding of DNA at the origin of DNA replication. RPA binding precedes loading of Pol α-primase and Pol α is bound after origin unwinding. Recruitment of DNA polymerases onto origins is also controlled by the Dpb11 protein, which apparently associates with the origins after the pre- RC assembly, since loading of Dpb11 is dependent on MCM and RPA. Loading of Pol α and Pol ε onto replication origins is dependent on Dpb11, suggesting that the association of Dpb11 with origins is a prerequisite for the assembly of a replicative complex in order to initiate cellular DNA replication.
iii. Elongation:
The elongation process is different for the 5-3′ and 3-5′ template. The 3-5′ proceeding daughter strand that uses a 5-3′ template- is called leading strand because DNA polymerase delta can read the template and continuously adds nucleotides complementary to the nucleotides of the template.
Lagging strand synthesis of Okazaki fragments is initiated in essentially the same way as leading strand synthesis: synthesis is primed by DNA polymerase α, a deoxynucleotide stretch is added by DNA polymerase a, and then switching to DNA polymerase δ takes place. Priming is a frequent event in lagging strand synthesis, with RNA primers placed every 50 or so nucleotides. About 10-nucleotide lengths of RNA primer are extended through addition of 10 to 20 deoxynucleotides by DNA polymerase a before DNA polymerase δ (or ε) enters. All but one of the ribonucleotides in the RNA primer are removed by RNase H1; then the exonuclease activity of the FEN1/RTH1 complex removes the one remaining ribonucleotide, and DNA ligase joins the Okazaki fragment to the growing DNA strand. Each new double helix is consisted of one old and one new chain. This is what we call semi-conservative replication (Fig. 7.13).
iv. Termination:
The last step of DNA replication is the termination. This process happens when the DNA polymerase reaches to an end of the strands. In the last section of the lagging strand, when the RNA primer is removed, it is not possible for the DNA polymerase to seal the gap (because there is no primer). So, the end of the parental strand where the last primer binds isn’t replicated. These ends of linear (chromosomal) DNA consist of noncoding DNA that contains repeat sequences and are called telomeres. As a result, a part of the telomere is removed in every cycle of DNA replication. The DNA replication is not completed before a mechanism of repair fixes possible errors caused during the replication. Enzymes like nucleases remove the wrong nucleotides and the DNA polymerase fills the gaps.
Essay # 6. Replicating the Ends of Chromosomes—Telomeres and Telomerase:
Telomeres are short (5-8 bp), tandemly repeated, G-rich nucleotide sequences that form protective caps 1-12 kbp long on the ends of chromosomes. Vertebrate telomeres have a TTAGGG consensus sequence. Telomeres are necessary for chromosome maintenance and stability. DNA polymerases cannot replicate the extreme 5′-ends of chromosomes because these enzymes require a template and a primer and replicate only in the 5′ —> 3′ direction.
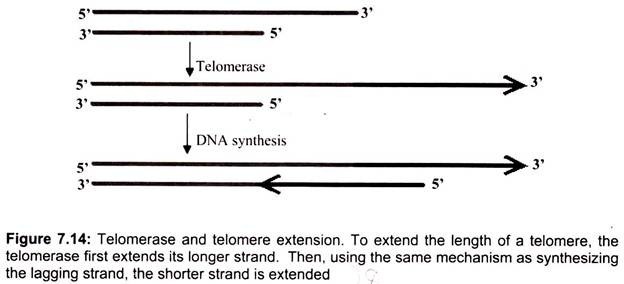
Thus, lagging strand synthesis at the 3′-ends of chromosomes is primed by RNA primase to form Okazaki fragments, but these RNA primers are subsequently removed, resulting in gaps in the progeny 5′-terminal strands at each end of the chromosome. Telomerase, an RNA -dependent DNA polymerase (126 kD) whose catalytic subunit show substantial homology to other reverse transcriptases, maintains telomere length by restoring telomeres at the 3′- ends of chromosomes. Telomerase is a ribonucleoprotein, and its RNA component contains a 9- to 30-nucleotide-long region that serves as a template for the synthesis of telomeric repeats at DNA ends.
The telomerase contains an essential RNA component which is complementary to the telomere repeat sequence. Hence, the internal RNA can serve as the template for synthesizing DNA. Through telomerase translocation, a telomere may be extended by many repeats. The human telomerase RNA component is 450 nucleotides long; its template sequence is CUAACCCUAAC. Telomerase uses the 3′-end of the DNA as a primer and adds successive TTAGGG repeats to it, employing its RNA as template over and over again. Bacteria do not have the end-replication problem, because its DNA is circular. In eukaryotes, telomeres help to protect chromosomes from fusing with each other and to solve the end- replication problem. In the absence of telomerase, the telomere will become shorter after each cell division. When it reaches a certain length, the cell may cease to divide and die. Therefore, telomerase plays a critical role in the aging process.
When the polymerase reaches the end of replication, there is another problem due to the antiparallel structure. The RNA primer on the leading strand occupies a small portion of the DNA, which is not exposed to polymerase and therefore is not copied. As a result, there would be a gap on the newly duplicated DNA at the original leading strand on the 5′ end of non-circular (viz. eukaryotic) chromosomes. The sticking out 3′ end consists of noncoding DNA called the telomere, which can be simply cut off (Fig. 7.14). Before the DNA replication is finally complete, enzymes are used to proofread the sequences to make sure the nucleotides are paired up correctly. If mistake or damage occurs, an enzyme called nuclease will remove the incorrect DNA. DNA polymerase will then fill in the gap.
Essay # 7. Post-Replicative Modification of DNA: Methylation:
One of the major post-replicative reactions that modify the DNA is methylation. DNA methylation is universal in bacteria, plant, and animal. DNA methylation is a type of chemical modification of DNA that are stable over rounds of cell division but do not involve changes in the underlying DNA sequence of the organism. Chromatin and DNA modifications are two important features of Epigenetics and play a role in the process of cellular differentiation, allowing cells to stably maintain different characteristics despite containing the same genomic material. However, the DNA methylation level is dynamic over the course of development in multicellular organisms.
Methylation of DNA in prokaryotic cells also occurs. The function of this methylation is to prevent degradation of host DNA in the presence of enzymatic activities synthesized by bacteria called restriction endonucleases. In prokaryotic organisms, DNA methylation occurs at the number 5 carbon of the cytosine pyrimidine ring and the number 6 nitrogen of the adenine purine ring.
However, in eukaryotic organisms DNA methylation occurs only at the number 5 carbon of the cytosine pyrimidine ring. In mammalian, DNA methylation occurs most at the number 5 carbon of the cytosine of a CpG dinucleotide. “CpG“ is shorthand for “—C—phosphate —G—”, that is, cytosine and guanine separated by a phosphate, which links the two nucleosides together in DNA.
The role of methylation in eukaryotic DNA serves two clearly defined and overlapping functions. The methylation of CpG dinucleotides affects the overall structure of chromatin which in turn broadly alters the availability of the chromatin to the transcriptional machinery. This effect of methylation is one mechanism of epigenesis. Epigenetics is defined as the study of the mechanism that produces phenotypic effects from gene activity during differentiation and development, or heritable changes in gene expression that do not involve changes in gene sequence.
CpG dinucleotide is only 1% in human genome, which is great fewer than expected. Between 70-80% of all CpGs are methylated. Unmethylated CpGs are grouped in clusters called “CpG islands” that are present in the 5′ regulatory regions of many genes. In many disease processes such as cancer, gene promoter CpG islands acquire abnormal hypermethylation, which results in heritable transcriptional silencing. DNA methylation may impact the transcription of genes in two ways.
First, the methylation of DNA may itself physically impede the binding of transcriptional proteins to the gene, thus blocking transcription. Second, and likely more important, methylated DNA may be bound by proteins known as Methyl- CpG-binding domain proteins (MBDs). MBD proteins then recruit additional proteins to the locus, such as histone deacetylases and other chromatin remodeling proteins that can modify histones, thereby forming compact, inactive chromatin termed silent chromatin. This link between DNA methylation and chromatin structure is very important. In particular, loss of Methyl-CpG-binding Protein 2 (MeCP2) has been implicated in Rett syndrome and Methyl- CpG binding domain protein 2 (MBD2) mediates the transcriptional silencing of hyper- methylated genes in cancer.