In this essay we will discuss about the genetics of development of drosophila, explained with the help of suitable diagrams.
General Body Plan:
The role genes play in determining the general axes of the body plan has been worked out at several levels. First, mutations causing female sterility were isolated. (C. Nusslein-Volhard and E. Weischans (1980) were instrumental in systematically isolating many of these mutants).
For example, among normal female flies that were sterile, some produced embryos without heads or thoracic structures. The gene for this mutation is called bicoid. It codes for a specific transcription factor, the Bicoid protein.
A student of Genetics/Molecular Biology should remember that gene names are italicized, using the first letter, lower case for recessive and upper case for dominant; the protein product of these genes is not italicized, but the first letter is capitalized.
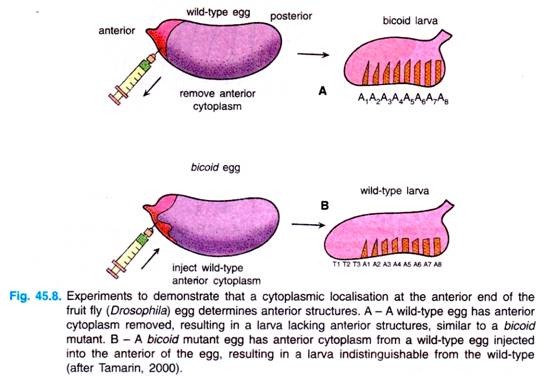
Pricking the anterior end of a normal embryo, causing the loss of cytoplasm from that end (Fig. 45.8), can mimic (resemble) these mutants. This experiment indicated that there is some cytoplasmic localisation determining the development of the anterior end of the fly.
To support that idea further, it was possible to get normal development from a bicoid fly by injecting the anterior end of biocoid embryo with cytoplasm from a normal embryo (Fig. 45.8B). This process of facilitating normal development by manipulating the embryo is termed as rescue experiment.
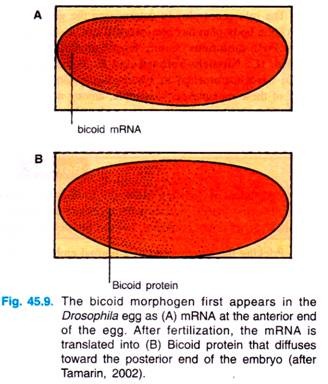
By probing with a complementary oligonucleotide to the bicoid messenger RNA (=mRNA), researchers found that the bicoid mRNA is formed in the nurse cells and then passed into oocyte, where this mRNA becomes localized at the anterior tip (Fig. 45.9).
After fertilization, this mRNA is translated into Bicoid, which begins to diffuse from the anterior end of the egg, until it reaches about 50% of the length of the egg. The protein can be visibly located by treating the egg with antibodies to the protein; these antibodies can then themselves be made visible (Fig. 45.9).
The Bicoid protein is called a morphogen, a substance that diffuses through the egg and by its concentration determines the developmental fate of that part of the embryo. Since maternal cells, not the embryo itself, produce this morphogen, the gene responsible for its production is called a maternal-effect gene.
Other maternal-effect genes are involved in formation of the anterior pattern that produces headless embryos. However, they do not appear to produce a morphogen. Rather, these genes seem to be involved in transport, stabilisation and modification of these other genes (i.e., swallow. exuperantia), Bicoid protein is found in the nurse cells but not in the embryo; cytoplasm from the nurse cells of these mutants can rescue bicoid mutants, indicating that the morphogen is present but not delivered to the oocyte. Only mutants of the bicoid gene itself cannot rescue the various headless mutants because only in bicoid mutants is the morphogen itself missing.
Researchers found that in Drosophila one set of gene encoded gradient morphogens for the anterior part of the embryo, another set of genes encoded morphogen responsible for organising the posterior region of the embryo, and a third set of genes encoded proteins that produced the terminal region at both ends of the embryo. For example, four maternal mRNAs are critical to the formation of the anterior-posterior axis.
1. The bicoid and hunchback mRNAs, whose protein products are critical for head and thorax formation.
2. The nanos and caudal mRNAs, whose protein products are critical for the formation of the abdominal segments.
The bicoid mRNAs are located in the anterior portion of the unfertilized egg, and are chained to the anterior microtubules.
The nanos mRNAs are bound to cytoskeleton in the posterior region of the unfertilised egg.
The hunchback and caudal mRNAs are distributed throughout the oocyte.
Through experiments similar to ones described for bicoid, four independent signalling pathways of maternal-effect genes have been isolated. These pathways determine the general body plan of the developing embryo: anterior, posterior, terminal and dorso-ventral.
The posterior pattern is controlled by the gradients of a protein, called Nanos. Before the nanos gene is active, producing mRNA, the first posterior gene is active, is Oskar; the localisation of Oskar mRNA then defines the localisation of nanos mRNA.
Mutant embryos can be rescued by wild-type-cytoplasm; the nanos mRNA is localised at the posterior tip of the embryo and produces a protein that diffuses from that tip. Maternal-effect genes that act in a somewhat different manner control the other two pattern systems in the developing embryo (i.e., terminal and dorso-ventral patterns).
The terminal pattern controls development of both ends of the embryo; a key gene is torso. This gene codes for a membrane-bound tyrosine kinase receptor protein that is found evenly distributed on the outer surface of the developing embryo. (Note. Tyrosine kinase phosphorylate the amino acid tyrosine in specific proteins).
Apparently, other genes in follicle cells located only at the poles of the egg produce a substance that activate torso tyrosine kinase receptor, making it active in only the poles of the egg (Fig. 45.10).
A material-effect gene, Toll controls the dorso-ventral pattern of the embryo; this gene also produces a membrane receptor. Thus, four pathways of maternal effect genes determines the major body plan of the egg.
Two of the pathways are determined by genes that result in diffusion of a morphogen (bicoid and nanos) and two are determined by genes for membrane receptors. About thirty maternal effect genes are known.
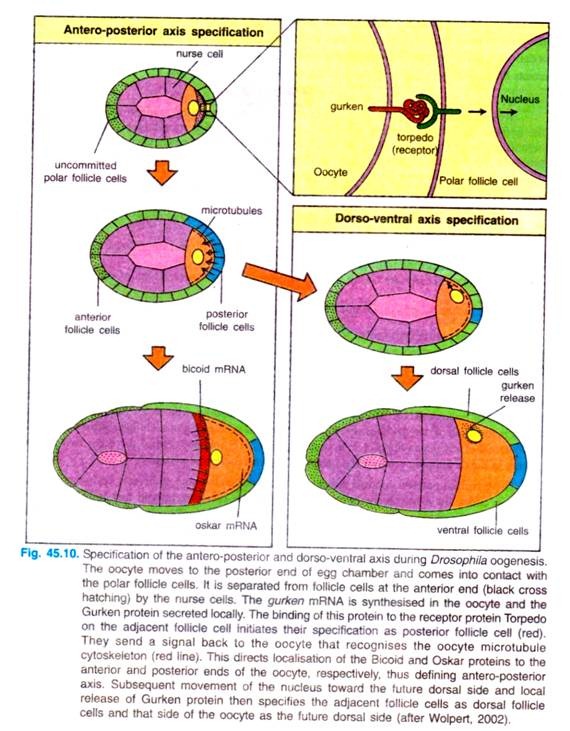
Interaction of oocyte and follicle cells for the determination of antero-posterior and dorso-ventral axes. The first visible sign of antero-posterior polarisation during oogenesis is the movement of the oocyte towards one end of the egg chamber, when it comes into contact with follicle cells (Fig. 45.10).
The localisation of the oocyte requires cadherin-dependent adhesion between the two cell types. Both the oocyte and the posterior follicle cells express higher levels of the adhesion molecule, the E-cadherin is removed, the oocyte is randomly positioned.
The oocyte then induces these follicle cells to adopt a posterior fate, while the follicle cell at the other end of the egg chamber, which are not in contact with oocyte, remain unaffected and become the anterior follicle cells. The inductive signal from the oocyte is transmitted by the Gurken protein.
The Gurken protein is synthesized and secreted by the oocyte at the posterior end. where the oocyte nucleus is located at the time. It binds to a receptor protein in the follicle cell plasma membrane encoded by torpedo gene. The Torpedo protein is a trans-membrane receptor tyrosine protein.
The posterior follicle cells send a signal back to the oocyte’s microtubule cytoskeleton into an array of microtubules stretching from the anterior end towards the posterior end. The microtubule organisation is disrupted in par-1 mutants. Microtubule reorganisation is essential for the localisation of bicoid mRNA at the anterior end of the egg.
The bicoid mRNA is made by nurse cells located next to the anterior end of the developing oocyte, and is transferred from them to the egg. The bicoid mRNA interacts with the microtubule array in such a way that it is moved toward the anterior end and retained there.
Similarly, oskar mRNA, which specifies the egg posterior germplasm that gives rise to the germ cells, is delivered into the oocyte by nurse cells and moved to the posterior end through its interaction within the microtubule array.
The nanos inRNA is also localised to the posterior end. Several maternal genes are necessary for the localisation of bicoid mRNA. For example, mother flies mutant for gene exuperantia have eggs in which the bicoid mRNA is distributed throughout the egg and not restricted to the anterior. Therefore, exuperantia gene must be involved in the anterior localisation process.
The setting-up of the egg’s dorso-ventral axis involves a later set of oocyte-follicle cell interactions, which occur after the posterior end of the oocyte has been specified and which depend on the previous reorganisation of the microtubule array. The oocyte nucleus moves along the microtubules from the posterior of the oocyte to a site on the anterior margin. In this new position, gurken gene is expressed in the oocyte nucleus again.
This time, the locally secreted Gurken protein acts as a signal to adjacent follicle cells on one side of the oocyte, specifying them as dorsal follicle cells; the side away from the nucleus thus becomes the ventral region by default. The ventral follicle cells produce proteins, such as pipe, that are only deposited in the ventral vitelline envelope.
Zygotic Genes:
Maternal-effect genes are the first in a series controlling a cascade of gene expression that eventually determines the fate of individual cells in the developing fly embryo. The rest of the genes are zygotic gens, genes active in the cells of embryo itself.
As we move down this cascade of genes, we go from broad patterns to more and more focussed gene activity. We go from a single cell with gradients of morphogens to stripes of cells with different active genes.
Segmentation Genes:
In Drosophila, the process of cell fate commitment appears to have two steps: specification and determination. Early in development, the fate of a cell depends on environmental signals such as those provided by the protein gradients mentioned above.
This specification of cell fate is flexible and can still be altered in response to signals from other cells. Eventually, the cells undergo transition from this loose type of commitment to an irreversible determination. At this point, the fate of a cell becomes cell intrinsic. The transition from specification to determination in Drosophila is mediated by the segmentation genes.
Thus, once the general body plan of Drosophila is in place, development continues in the formation of parasegments and then segments. The various organs of the fruit fly’s body are produced from these segments. Further development is now under the control of the zygote’s own genes, generally referred to segmentation genes.
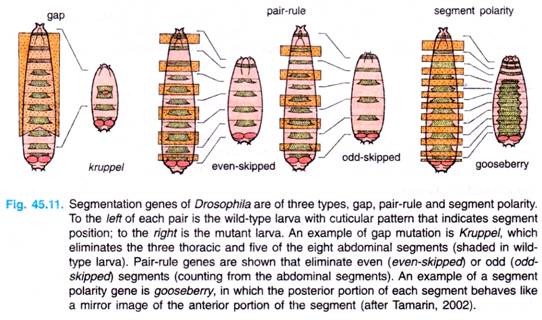
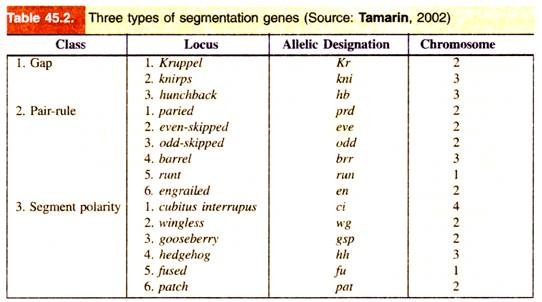
These genes are of following three types: gap genes, pair rule genes and segment-polarity genes (Table 45.2, Fig. 45.11). These genes are activated sequentially, each by the genes activated before it; each group controls a smaller and more focussed domain of the fly’s development.
The maternal effect genes of the anterior-posterior system have created Bicoid and Nanos protein gradients. The segmentation genes add, narrow and focus these gradient signals until fourteen distinct bands form, corresponding fourteen parasegments that develop, creating compartments, from which arise a variety of tissues such as wings, legs and bristles.
Discovery of gap genes were made from studies of mutants (Drosophila) which has missing segments in the embryo (Fig. 45.11). The gradients of Bicoid and Nanos proteins act in gap genes, specifically hunchback.
Although the Hunchback protein is present in the egg from maternal production, the maternally supplied quantity is apparently not significant. Bicoid and Nanos independently create a Hunchback gradient that is maximal at the anterior end of the embryo, due to activation of Bicoid, and absent at the posterior end, due to Nanos repression.
Bicoid is a specific transcription factor that can bind to at least six sites in the promoter region of the hunchback gene. Three of these sites are strong binding sites and three are weak. Thus, depending on the concentration of Bicoid protein in the gradient, different levels of Hunchback protein are produced, creating the Hunchback gradient.
Experiments with extra copies of the bicoid gene show that it is the actual quantity of Bicoid protein present at a particular point, and not the shape of the gradient that actually determines the effect. Most probably, as more Bicoid is present, it binds to more of the hunchback promoter sites, resulting in greater transcriptional activity.
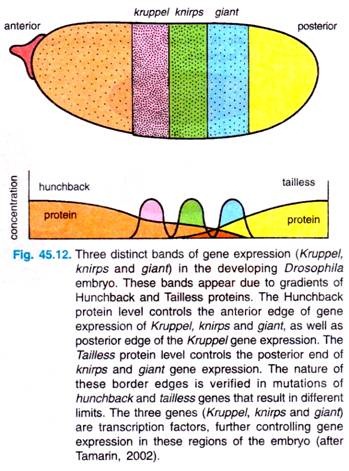
Various investigations have established that at least three gap genes are controlled by the concentrations of the specific transcription factor hunchback: Kruppel, knirps and giant. In response to the Hunchback gradient, these three genes are expressed in distinct stripes in the embryo (Fig. 45.12).
Both anterior and posterior edges of the Kruppel stripe are controlled by Hunchback concentration; Hunchback concentration also controls the anterior edges of knirps and Giant stripes. The posterior edges of Knirps and Giant stripes are controlled by the gradients of the Tailless protein, which is controlled in turn by the terminal maternal effect gene, torso (Fig. 45.12).
Investigators could know about the distributions of these proteins by antibody studies, and we know the limits of the protein distributions from studies of various mutants that lack the distinct edges of the stripes.
For example, the borders of the Kruppel protein stripe are changed in hunchback mutants in accordance with the number of copies of the genes. Investigators have thus gone from very broad and fuzzy regions of maternal-effect gene products to more defined bonds of gap gene products.
Interaction of gap gene proteins then controls transcription of the pair-rule genes (Fig. 45.11). These genes affect alternate sets of segments, even and odd. For example, mutants of even skipped gene cause the loss of the even-numbered segments, counting by the abdominal segments (loss of two thoracic segments as well as abdominal segments 2, 4, 6 and 8).
Finally, the segment-polarity genes are controlled by the pair-rule genes, resulting in genes that affect all segments (Fig. 45.11). For example, mutants of the gooseberry gene modify the posterior half of each segment, making it the mirror image of the anterior half.
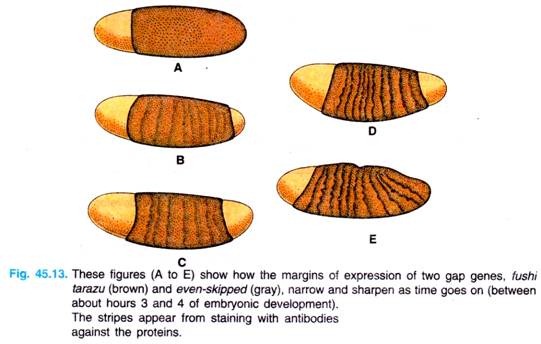
As development proceeds, and different classes of segmentation genes are activated, the borders of stripes of activation for these various genes become sharper and sharper, until cell-cell interactions focus the expression of different genes to neighboring cells.
For example, we see in Fig. 45.13 the narrowing and sharpening of the even-skipped and fushi tarazu bands in the developing embryo. (Note. The gene fushi tarazu, meaning, “not enough segments” in Japanese and is a pair-rule gene).
From molecular biological viewpoint, most segmentation genes are specific transcription factors, genes that interact with DNA to activate or repress transcription. Thus, pattern formation in development is a process of activating different genes in sequence, gradually narrowing the scope of which cells express a particular gene. There is one final group of genes we will discuss in development cascade in Drosophila, these genes, called homeotic genes, take control of the development of the segments.
Homeotic Selector Genes (Homeotic Mutants):
Homeotic Genes
After the segmental boundaries have been established in Drosophila embryo, the characteristic structures of the each segment are specified. This specification is accomplished by the homeotic selector genes.
Homeo means “similar”. Homeosis means transformation of a whole segment or structure into another related one. Homeotic mutants are mutants in which one structure is replaced by another (e.g., an antenna is replaced by a leg in case of Antennapedia gene of Drosophila). The homeotic genes are genes whose mutations can cause such transformations; thus, they are genes that specify the identity of a particular body segment.
In homeotic mutants, one cell type follows the developmental pathway other cell type normally follow. These genes define the future development of segments based on the pattern of expression of the segmentation genes before them.
When they mutate, they switch the development of that segment to an adjacent segment, usually anterior to it. Homeotic genes are also called memory genes because they set the developmental fate of a segment, a fate that is “remembered” from one cell division to the next. They are also called master-switch genes, since they control the activity of many other genes.
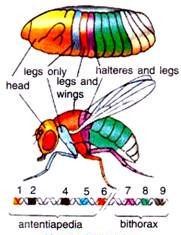
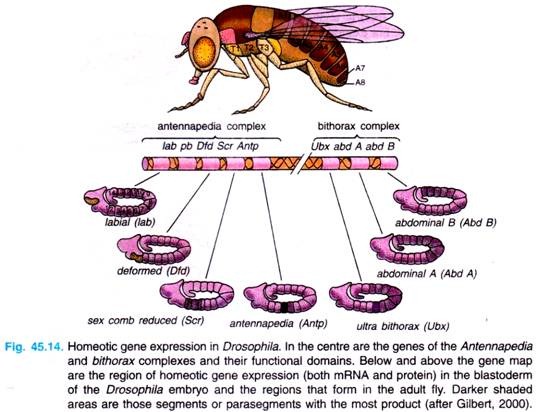
There are following two regions of chromosome 3 of Drosophila melanogaster that contain most of these homeotic genes (Fig. 45.14).
1. The first region of homeotic genes is the bithorax complex (BX-C) analysed extensively by E. Lewis (1978), D. Hogness and their colleagues. There are three protein-coding genes in this complex: ultrabithorax (ubx), which is required for the third thoracic segment; and the abdominal A (abdA) and abdominal B (abd B) genes, which are responsible for the segmental identities of the abdominal segment.
2. The second region, the Antennapedia complex (ANT-C) was worked extensively by T. Kaufman et al., (1990), W. Gehring (1994, 1999) and Waldmoto et al., (1998). This gene complex contains the homeotic genes labial (lab), Antennapedia (Antp), Sex comb reduced (Ser), proboscipedia (pb) and Deformed (Dfd).
The labial and Deformed genes specify the head segments, while the sex comb reduced and Antennapedia contribute to give the thoracic segments their identities. The proboscipedia gene appears to act only in adults, but in its absence, the labial palps of the mouth are transformed into legs.
The lethal phenotype of the triple-point mutants Ubx–, abdA–, and abdB is identical to that resulting from a deletion of the entire bithorax complex (BX-C). The region containing both the Antennapedia complex (ANT-C) and the bithorax complex (BX-C) is often referred to as homeotic complex (Hom-C).
Because these genes are responsible for the specification of fruit fly body parts, mutations in them led to bizarre phenotypes. In 1894, William Bateson called these organisms “homeotic mutants”, and they have fascinated developmental biologists for decades.
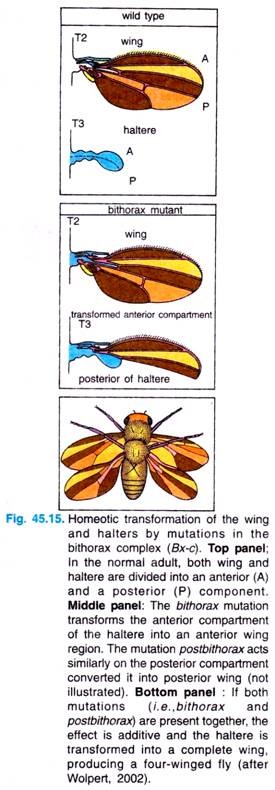
For example, the body of normal adult fly contains three thoracic segments, all of which produce a pair of legs. The first thoracic segment does not produce any further appendages, but the second thoracic segment produces both a set of legs and a set of wings.
The third thoracic segment produces a set of wings and a set of balancers known as halters. In homeotic mutants, the specific segmental identities can be changed. For example, when the bithorax complex (BX-C) gene is deleted, the third thoracic segment becomes transformed into another second thoracic segment. The result is a fly with four wings (Fig. 45.15).
Similarly, the Antennapedia protein is usually used to specify the second thoracic segment of the fly. But when flies have a mutation wherein the Antennapedia is expressed in the head (as well as in the thorax), legs than antennae grow out of the head sockets. In the recessive mutant of Antennapedia, the gene fails to be expressed in the second thoracic segment, and antennae sprout out of the leg positions.
Both complexes, i.e., the Antennapedia (ANT-C) and bithorax (BX-C) are remarkable in that gene order on the chromosome corresponds to the spatial and temporal order of gene expression along the body. The selector genes of the two complexes are expressed in the ectoderm and in the underlying somatic and visceral mesoderm.
They are not expressed in the endoderm from which the gut develops. However, induction of the endoderm by visceral mesoderm seems to transfer to the endoderm some of the segment specific pattern conferred on the mesoderm by selector gene activity.
Thus, the genes in these homeotic complexes are arranged in order of their progressive action from anterior to posterior on the fly. One model of action for these genes suggests that they require the action of genes of the adjacent anterior segment plus the action of that homeotic gene itself. In this way, loss of function of a particular gene by mutation would cause a segment to develop like the previous section in the anterior direction.
The Homeo Box:
Using recombinant DNA techniques, Gehring and his colleagues (1994) found a consensus sequence of 180 base pairs of DNA in genes of the Antennapedia and bithorax complexes. Further, probing localised this same segment of 180 base pairs to about a dozen genes in Drosophila, all with homeotic or segmentation properties.
They thus called this DNA sequence the homeo box. The nucleotides of the homeo box are translated into a peptide region of 60 amino acids called the homeo domain, i.e., the DNA binding helix-turn-helix like domain.
The Homeodomain
Using a recombinant probe for the homeo box or a computer search for the consensus sequence, researchers found it in the genes of plants (i.e., Arabidopsis, Antirrhinum), yeast, sea urchins, honey bees, frogs, toads (Xenopus laevis), mice and human beings.
This high degree of sequence conservation across divergent groups of organisms indicate that the sequence is crucial to the functioning of homeotic genes and that the mechanism arose early in evolutionary time.
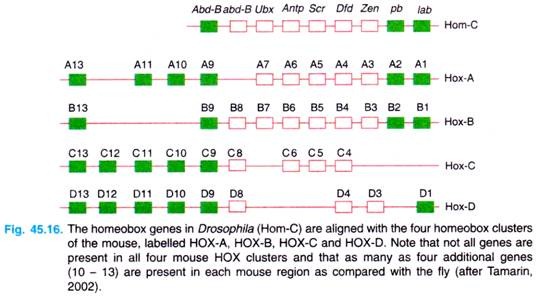
In fact, Walldorf, Fleig and Gehring (1989) isolated seven homeo box-containing genes from the honey bee (Apis mellifera) and found that six of the seven genes contained homeo boxes with greater than 90 per cent sequence identity with the homologous homeo box in D. melanogaster (see Gardner et al, 2(X)1). There are four homeotic clusters in mice, called Hox clusters, on four different chromosomes (Fig. 45.16).
There are at least nine homeo box genes in human chromosome 17 (the HOX B genes) which appear to determine the structure of the central nervous system. Similarly, the HOX D cluster plays a role in development of limbs.
With multiple copies, the genes could be modified by evolution while still maintaining one copy functioning as originally intended. This duplication has allowed great complexity in higher eukaryotes.
Further, as the Drosophila melanogaster homeobox genes function from anterior to posterior, so do the homeobox genes in other organisms. The conservation of these homeobox sequence among higher animal species suggests that they play important roles, probably in regulating some aspects of morphogenesis.