Are you looking for an essay on ‘Nucleic Acid’? Find paragraphs, long and short essays on ‘Nucleic Acid’ especially written for school and college students.
Essay on Nucleic Acid
Essay Contents:
- Essay on the Introduction to Nucleic Acids
- Essay on the Chemical Composition of Nucleic Acids
- Essay on the Formation of DNA and RNA Polymer
- Essay on the Types of Nucleic Acid
- Essay on the Architects behind the Discovery of DNA
- Essay on the Summary of Nucleic Acid
1. Essay on the Introduction to Nucleic Acids:
Nucleic acids are complex compounds of monomeric nucleotides, composed of phosphoric acid, sugar and nitrogenous base and involved in the preservation, replication, and expression of hereditary information in every living cell. Nucleic acids were so named because they were first found in the nucleus of cells.
Johann Friedrich Miescher in 1870 isolated a weakly acidic substance of unknown function in the nuclei of human white blood cells, and named this material “nuclein“, which later came to known as DNA. In the 1920s nucleic acids were found to be major components of chromosomes, small gene-carrying bodies in the nuclei of complex cells. Elemental analysis of nucleic acids showed the presence of phosphorus, in addition to the usual C, H, N and O.
It was later found that there were two kinds of nucleic acids, according to the bases that were identified. One type of nucleic acid was obtained from animal glands and later called DNA, while the other type was obtained from yeast cells, and called RNA. It was not until the 1940s that biochemists realized that both DNA and RNA are present in all living cells, whether plant or animal. American chemist Marshall Nirenberg was later credited with translating the code of life and was awarded the Nobel Prize in 1968. He demonstrated that RNA could be translated into protein.
Initially, it was thought that there was only one kind of RNA, but other types of RNAs with specialized functions were later discovered. On the basis of presence of deoxyribose sugar, chromosomal nucleic acids were called deoxyribonucleic acids, abbreviated as DNA. Analogous nucleic acids, RNA, in which the sugar component is ribose, termed ribonucleic acids. The acidic character of the nucleic acids was attributed to the phosphoric acid moiety.
2. Essay on the Chemical Composition of Nucleic Acids:
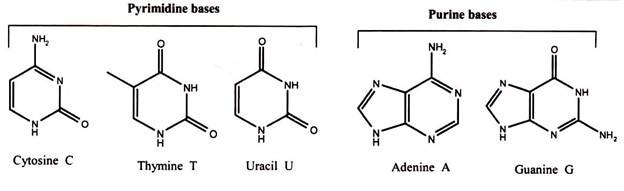
The basic structure of nucleic acids consists of bases, sugar and phosphates. There are three monocyclic bases, cytosine, thymine and uracil, called pyrimidines, and the two bicyclic bases, adenine and guanine, called purines. It can be easily remembered by the notation the longer word (pyrimidine) represents the smaller structure (only one ring), and vice versa. Each has at least one N-H site at which an organic substituent may be attached.
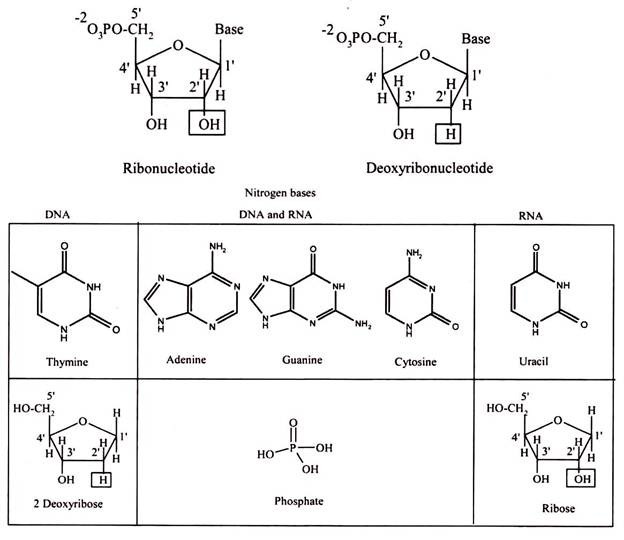
Nucleic acids are polynucleotide, formulated as alternating copolymers of phosphoric acid (P) and nucleosides (N). Nucleosides consist of sugar and bases. The base – sugar covalent bond in a nucleoside or nucleotide is an N-glycosidic link because it involves the N of the purine or pyrimidine ring. The sugar that is part of a nucleotide is a 5-carbon atom sugar in its ring form. It will either be ribose in RNA or deoxyribose in DNA.
The “de-oxy” simply means that the ribose molecule has lost oxygen from the second carbon, so the more correct name for deoxyribose is 2-deoxyribose. As the last asymmetric carbon atom has an OH to the right, these molecules are sometimes given the more complete names of D-ribose and also D -2-deoxyribose.
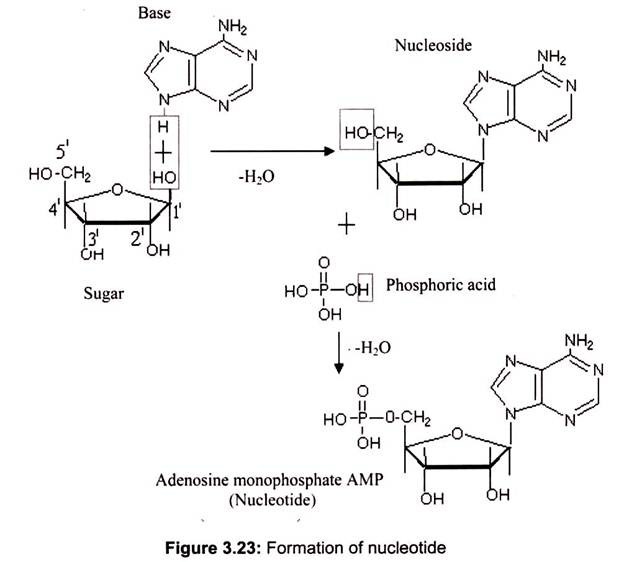
Nucleosides are formed by the N-glycosidic link between sugar and nitrogen base. The phosphate groups are linked to the sugars at the 5′ position. The addition of one to three phosphate groups generates a nucleotide, also known as a nucleoside monophosphate, nucleoside diphosphate, or nucleoside triphosphate (Fig. 3.23).
3. Essay on the Formation of DNA and RNA Polymers:
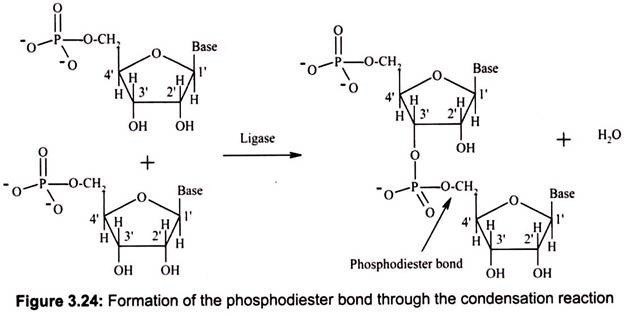
DNA and RNA polymers are synthesized by forming phosphodiester bonds between nucleotides (Fig. 3.24). In this arrangement, a phosphate group acts as a bridge between the 5′ position of one sugar and the 3′ position of the next. This arrangement is called the “sugar- phosphate backbone” of DNA or RNA; the bases hang off to the side.
In the cell, DNA or RNA polymers are synthesized using nucleoside triphosphate monomers as precursors. During polymer synthesis, two of the phosphate groups of the incoming nucleoside triphosphate are cleaved off, and this provides the energy needed to power the reaction. The remaining phosphates take its place in the sugar-phosphate backbone of the growing nucleic acid chain and a pyrophosphate molecule (two linked phosphates) is released.
During DNA replication, it always moves from the 5′ end to the 3′ end, and the incoming triphosphate joins the 3′ end of the chain. Transcription (RNA synthesis from a DNA gene) also moves in this 5′-to-3′ direction. The 5′ end is considered the “upstream” end of the gene, and it is the end on which the gene promoter (the transcription initiator) is located. In a nucleic acid chain, two nucleotides are linked by a phosphodiester bond, which may be formed by the condensation reaction similar to the formation of the peptide bond. However, the whole nucleic acid chain is usually synthesized by RNA polymerase or DNA polymerase.
Like peptide chains, a nucleic acid chain has also orientation: its 5′ end contains a free phosphate group, while 3′ end contains a free hydroxyl group. Synthesis of a nucleic acid chain always proceeds from 5′ to 3′. Therefore, unless specified otherwise, the sequence of a nucleic acid chain is written from 5′ to 3′ (left to right).
In DNA or RNA, a nucleic acid chain is also called a strand. A DNA molecule typically contains two strands, whereas most RNA molecules contain a single strand. The length of a nucleic acid chain is represented by the number of bases. In the case of a double-stranded nucleic acid, bases are paired between two strands. Therefore, its length is given by the number of base pairs (bp). 1 kb = 1000 base pair; 1 Mb = 1 million bases. Oligonucleotides refer to short nucleic acid chains (< 50 bases or bp) while polynucleotides have longer chains.
4. Essay on the Types of Nucleic Acid:
There are two types of nucleic acids:
A. Deoxyribonucleic Acid (DNA)
B. Ribonucleic Acid (RNA)
A. Deoxyribonucleic Acid:
Deoxyribonucleic Acid (DNA) is the genetic material found in the cells of all living organisms. DNA is the fundamental molecule which carries the genetic instructions used in the development and functioning of all known living organisms and some viruses. The main role of DNA molecules is the long-term storage of information. DNA is often compared to a set of blueprints or a recipe, or a code, since it contains the instructions needed to construct other components of cells, such as proteins and RNA molecules. The DNA segments that carry the genetic information are called genes, but other DNA sequences have structural purposes, or are involved in regulating the use of genetic information.
Oswald Avery and colleagues in 1944 demonstrated that bacterial DNA was likely the genetic agent that carried information from one organism to another in a process called “transformation“. On the basis of careful analysis of DNA from many sources, Erwin Chargaff found that the composition of DNA is species specific. In addition, he found that the amount of adenine (A) always equaled the amount of thymine (T), while the amount of guanine (G) always equaled the amount of cytosine (C), regardless of the DNA source, known as Chargaff s rule.
In a second critical study, Alfred Hershey and Martha Chase showed that when a bacterium is infected and genetically transformed by a virus, at least 80% of the viral DNA enters in the bacterial cell and at least 80% of the viral protein remains outside. Together with the Chargaff findings this work established that DNA is the repository of the unique genetic characteristics of an organism.
The DNA- Double Helix :
DNA is usually a double-helix and has two strands running in opposite directions. There are some examples of viral DNAs which are single-stranded. Each strand has a backbone made up of (deoxy-ribose) sugar molecules linked together by phosphate groups. All DNA strands are read from the 5′ to the 3′ end where the 5′ end terminates in a phosphate group and the 3′ end terminates in a sugar molecule.
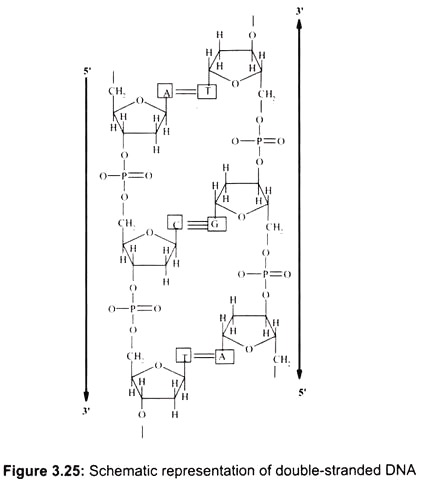
In the double-stranded DNA, the A-T base-pair has 2 hydrogen bonds and the G-C base- pair has 3 hydrogen bonds. The G-C interaction is therefore stronger (by about 30%) than A-T. The bases are oriented perpendicular to the helix axis. They are hydrophobic in the direction perpendicular to the plane of the bases (cannot form hydrogen bonds with water). The interaction energy between two bases in a double-helical structure is therefore a combination of hydrogen-bonding between complementary bases and hydrophobic interactions between the neighboring stacks of base-pairs (Fig. 3.25).
The backbone of polynucleotides is highly charged (1 unit negative charge for each phosphate group; 2 negative charges per base-pair). If there is no salt in the surrounding medium, there is a strong repulsion between the two strands and they will fall apart. Therefore, counter-ions are essential for the double-helical structure. Counter-ions shield the charges on the sugar-phosphate backbone.
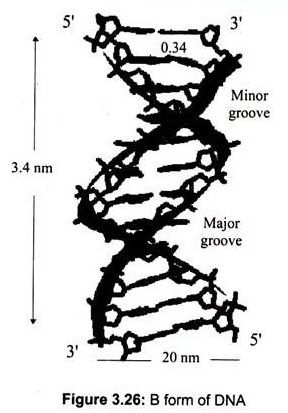
The most common DNA structure in solution is the B-DNA (Fig.3.26). The two strands form a “double helix” structure, was first discovered by James D. Watson and Francis Crick in 1953. In this structure, the B form, the helix makes a turn every 3.4 nm (34A°), and the distance between two neighboring base pairs is 0.34 nm (3.4A°). Hence, there are about 10 pairs per turn.
The intertwined strands make two grooves of different widths, referred to as the major groove and the minor groove, which may facilitate binding with specific proteins. The major groove is approximately 50% wider than the minor. Proteins that interact with DNA often make contact with the edges of the base pairs that protrude into the major groove. The chemical groups on the edges of GC and AT base pairs are those available for interaction in the major and minor grooves.
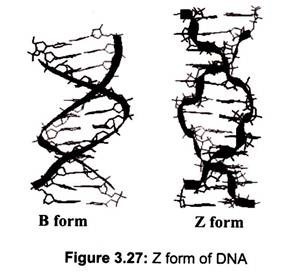
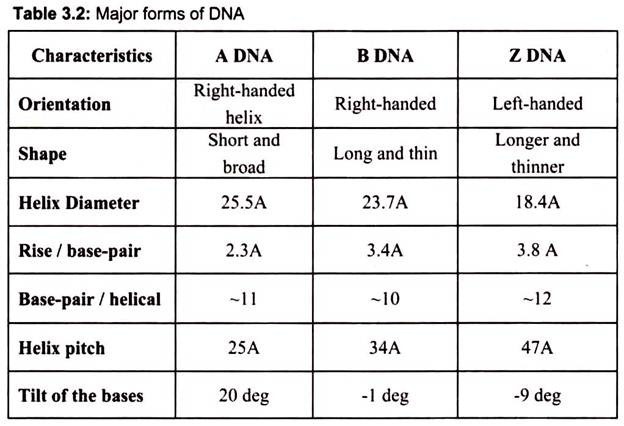
DNA can adopt several other conformations under conditions of applied force or twists, under low hydration conditions (Table 3.2). In a solution with higher salt concentrations or with alcohol added, the DNA structure may change to an ‘A’ form, which is still right- handed, but every 2.3 nm makes a turn and there are 11 base pairs per turn. Another DNA structure is called the Z form, because its bases seem to as zigzag. Z DNA is left-handed (Fig. 3.27). One turn spans 4.6 nm, comprising of 12 base pairs. The DNA molecule with alternating G-C sequences in alcohol or high salt solution tends to have such structure.
The A-form crystallizes under low hydration conditions and is not normally found in the cell. It is, however, the structure adopted by double-stranded regions in RNA as well as the transient double-helix between DNA and RNA during transcription. Both A- and B-DNA are right-handed helices whereas Z-DNA is a left-handed helix and is commonly found in the regions of DNA that have an alternating purine-pyrimidine (e.g. 5′-CGCGCGCG-3′ or 5′- CGCGCATGC-3′) sequences (Fig. 3.27).
The coding regions (the genes) in the DNA strand make up represent only a fraction of the total amount of DNA. The stretches that flank the coding regions are called introns, and consist of non-coding DNA. Introns were considered as junk in the early days. Today, biologists and geneticists believe that this non-coding DNA may be essential in order to expose the coding regions and to regulate how the genes are expressed.
Chromatin:
DNA is found associated with histones and non-histone proteins, to form the chromatin. There are 3 x 109 nucleotide pairs in the human haploid genome, representing about 30,000 genes dispersed over 23 chromosomes for a haploid set.
Histones are the major proteins of chromosomes, containing a high proportion of basic amino acids (arginine and lysine) that facilitate binding to the negatively charged DNA molecule. In addition, chromatin contains an approximately equal mass of a wide variety of nonhistone chromosomal proteins. Histones are not found in eubacteria (e.g., E. coli), although the DNA of these bacteria is found associated with other proteins that presumably function like histones to pack the DNA within the bacterial cell.
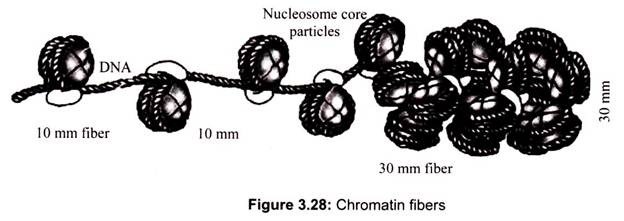
Archaebacteria, however, do contain histones that package their DNAs in structures similar to eukaryotic chromatin. The packaging of DNA into nucleosomes yields a chromatin fiber approximately 10 nm in diameter (Fig. 3.28). The chromatin is further condensed by coiling into a 30-nm fiber, containing about six nucleosomes per turn.
Chromatin is found in two varieties- euchromatin and heterochromatin. Originally, the two forms are distinguished cytologically by how intensely they stained the former is less intense, while the latter stains intensely, indicating their tighter packing. Heterochromatin is usually localized to the periphery of the nucleus.
(a) Heterochromatin:
Heterochromatin is a tightly packed form of DNA. Its major characteristic is that the transcription is limited; it is a means to control gene expression, through regulation of the transcription initiation. Heterochromatin is a genetically inactive region of chromosomes that either lack genes or contain genes that are repressed and replicates in S phase of the cell cycle and is found only in eukaryotes.
In some organisms heterochromatin can also be identified by association with small RNA molecules or high levels of DNA methylation. The histone tails can be modified in various ways (including acetylation, methylation, ubiquitination, poly-ADP-ribosylation and phosphorylation), and these modifications are believed to serve as signals as to whether a region will be packed into silent heterochromatin or will remain active as euchromatin. In some organisms heterochromatin can also be identified in association with small RNA molecules or high levels of DNA methylation.
Heterochromatin is believed to serve several functions, from gene regulation to the protection of the integrity of chromosomes; For example, naked double-stranded DNA ends would usually be interpreted by the cell as damaged DNA, triggering cell cycle arrest and DNA repair. However, telomeres, which act as constant triggers of a DNA damage response, are shielded from the DNA damage machinery, as they are packed into heterochromatin.
(b) Euchromatin:
Euchromatin comprises the most active portion of the genome within the cell nucleus and found in both eukaryotes and prokaryotes. It is a lightly packed form of chromatin, and is often under active transcription. The lighter staining of euchromatin is due to the less compact structure. It contains structural genes which replicate and transcribe during G1 and S phase of interphase. In particular, it is believed that the presence of methylated lysine on the histone tails acts as a general marker for euchromatin. In prokaryotes, euchromatin is the only form of chromatin present, which indicates that the heterochromatin structure evolved later along with the nucleus.
B. Ribonucleic Acid (RNA):
Ribonucleic acids are a class of nucleic acids characterized by the presence of the sugar ribose and the organic base uracil instead of thymine as in DNA. Most RNA molecules, including messenger RNA and transfer RNA, act as cellular intermediaries; that is, they convert the genetic information stored in DNA into the proteins. In some viruses, RNA also serves as the hereditary material.
It is transcribed from DNA by enzymes called RNA polymerases and further processed by other enzymes. The enzyme progresses along the template strand in the 3′ → 5′ direction, synthesizing a complementary RNA molecule with elongation occurring in the 5′ → 3′ direction. The DNA sequence also dictates where termination of RNA synthesis will occur.
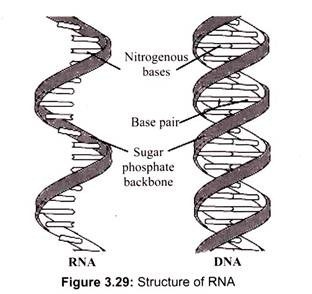
RNA, unlike DNA, is also found in other parts of the cell other than the nucleus. In fact, the majority of the RNA is present in the cytoplasm in various forms. Nuclear RNA is comprised of single-stranded sequences (DNA is double stranded) and has a lower molecular weight than DNA (Fig. 3.29).
The structure of RNA is almost similar to that of DNA but with a slight difference. The structural difference with DNA is that the RNA contains a -OH group both at the 2′ and 3′ position of the ribose ring, whereas DNA (which stands, in fact, for deoxy-RNA) lacks such a hydroxy group at the 2′ position of the ribose. The same bases can be attached to the ribose group in RNA as occur in DNA, with the exception that in RNA thymine does not occur, and is replaced by uracil, which has an H-group instead of a methyl group at the C-5 position of the thymine. Uracil is energetically less expensive to produce than thymine, which may account for its use in RNA. Thus, uracil is appropriate for RNA, where quantity is important but lifespan is not, whereas thymine is appropriate for DNA where maintaining sequence with high fidelity is more critical.
According to the “RNA world” hypothesis, RNA must have come before the first proteins, since without it there would have been no molecule of heredity and therefore no way for other molecules to have been manufactured consistently. RNA alone handled all of the tasks required for a cell to survive, acting both as a genetic material and a catalyst for the various reactions involved in metabolism and for its own assembly. Ribozymes, the catalytic RNA, that can act as their own enzymes, snipping themselves in two and splicing themselves back together. One possibility is that they are used to replicate RNA. Protoribosomes may have taken small strands of RNA from their surroundings and cut and pasted those which matched their template into a new, duplicate strand.
Unlike DNA, whose primary role is to store the genetic information, RNA plays many roles in the cell. RNA is structurally much more flexible than DNA because it is usually a mixture of single-stranded and duplex regions rather than predominantly duplex. The presence of the 2′-hydroxyl chemical group also makes RNA chemically more labile and not generally suitable as a molecule for storage of large amounts of genetic information over long periods of time.
RNA is readily cleaved in alkaline conditions and in the presence of divalent metal ions, both of which deprotonate the 2′ hydroxyl group, which then attacks the phosphate esterified at the 3′ position, resulting in chain scission and a 2′,3′ cyclic phos-phodiester terminal group.
Single-stranded regions of RNA are also very susceptible to attack by ribonucleases in the cell. Many RNAs, especially messenger RNAs, have a life time less than one cell division. This short life time is essential for cells to respond to changing environmental conditions. Other RNAs, such as ribosomal RNAs, tRNAs and small nuclear RNAs have considerable secondary structure and some are protected by complexes with proteins, stabilizing them further.
The three main functionally distinct varieties of RNA molecules are:
(1) Messenger RNA (mRNA) which is involved in the transmission of DNA information,
(2) Ribosomal RNA (rRNA) which makes up the physical machinery of the synthetic process, and
(3) Transfer RNA (tRNA) which also constitutes another functional part of the machinery of protein synthesis.
Types of RNA:
(a) Messenger RNA (mRNA):
Messenger RNA is RNA that carries information from DNA to the ribosome sites of protein synthesis in the cell. Once mRNA has been transcribed from DNA, it is exported from the nucleus into the cytoplasm (in eukaryotes mRNA is “processed” before being exported), where it is bound to ribosomes and translated into protein and get degraded into its component nucleotides, usually with the assistance of RNases.
As we know the information that mRNA carries is written in genetic code – a sequence of bases. Each code word is called a codon, a sequence of three adjacent nucleotides that specifies one of the twenty amino acids. There are 64 possible codons (4 × 4 × 4), and each codon codes for an amino acid. More than one codon can code for the same amino acid. For example, GGU, GGC, GGA, and GGG all code for the amino acid glycine. The codon AUG is the start codon and UAA, UAG, and UGA act as the end codon, codons which specify the end of a protein synthesis.
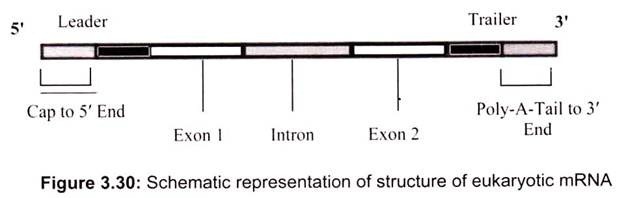
The mRNA in both prokaryotic and eukaryotic cells (Fig. 3.30) has three distinct regions, the 5′ Leader, gives physical space to ribosomes so that they can bind to mRNA and move down to the initiation codon, the coding region, is the part of mRNA that actually codes for the protein – the codons and 3′ trailer, which is simply a section that comes after the stop codons.
In eukaryotes, mRNA that has just been transcribed from DNA must undergo two post-transcriptional modifications before it can be translated. First is capping, a special methylated version of triphosphate guanine nucleoside is added to the 5′ end and addition of a poly-A-tail, a series of 50-150 adenine nucleotides, is added to the 3′ end.
It helps in the transportation of the mRNA out of the nucleus and determines the number of times mRNA can be translated before it is degraded. Prokaryotic mRNA lacks the methylated cap and the poly-a-tail, and there are no introns so it can be translated right after being transcribed.
Eukaryotic mRNA has regions which do not code for proteins (Fig. 3.30). These regions are called intervening sequences or introns. Regions which do code for something are called exons. After the primary mRNA transcript is produced, introns must be identified and removed by splicing with the aid of splicesomes, large RNA-protein complexes that contain many different enzymes and several kinds of RNA so that only exons remain. It is accomplished in the nucleus of a cell splicesomes which contain a short piece of RNA that complements base sequences found at either end of just about every intron.
(b) Transfer RNA (tRNA):
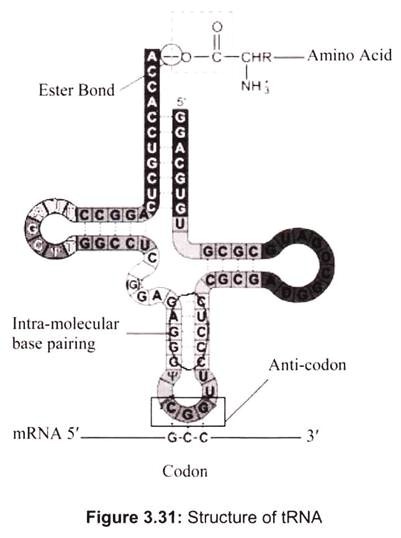
Transfer RNA is a small RNA chain of about 74-93 nucleotides that transfers a specific amino acid to a growing polypeptide chain at the ribosomal site of protein synthesis during translation. It has sites for amino-acid attachment and an anticodon region for codon recognition that binds to a specific sequence on the messenger RNA chain through hydrogen bonding. It is a type of non-coding RNA. The approximate shape of tRNA is known as clover leaf model, since tRNA has 3 loops and one stem (Fig. 3.31).
One end of the tRNA contains an anticodon loop which pairs with mRNA specifying a certain amino acid. The other end of the tRNA has the amino acid attached to the 3′ OH group via an ester linkage. tRNA with an attached amino acid is said to be “charged”. The enzyme that attaches the amino acid to the 3′-OH is called an aminoacyl tRNA synthetase. There is a specific tRNA for each amino acid, which are 20 in all.
Only the first 2 nucleotides in the tRNA anticodon loop are strictly required for the decoding of the mRNA codon into an amino acid. The third nucleotide in the anticodon is less stringent in its base-pairing to the codon, and is referred to as the “wobble” base, and the phenomenon is known as wobble hypothesis. Since the genetic code is degenerate, meaning that more than one codon can specify a single amino acid, the anticodon of tRNA can pair with more than one mRNA codon and still be specific for a single amino acid.
(c) Ribosomal RNA (rRNA):
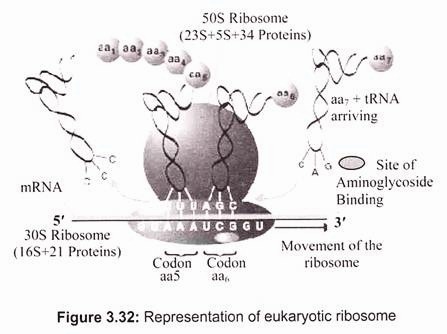
Ribosomal RNA (rRNA) is a component of the ribosomes, the protein synthetic factories in the cell. Eukaryotic ribosomes contain four different rRNA molecules- 18S, 5.8S, 28S, and 5S rRNA, where ‘S’ is the sedimentation coefficient (Svedberg unit) or density unit that is used in describing the results of ultracentrifugation and reflects the size and shape of a molecule or a particle (1S= 1 × 10-13 cm/sec/dyne). The larger the value of S, the bigger the particle is. Three of the rRNA molecules are synthesized in the nucleolus, and one is synthesized elsewhere. rRNA molecules are extremely abundant. They make up at least 80% of the RNA molecules found in a typical eukaryotic cell. rRNA forms the skeleton of ribosomes. The remainder of the ribosomes is comprised of proteins made in the cytoplasm. They enter the nucleus and then the nucleolus and finally join rRNA. The assembly of ribosomes is completed in the cytoplasm (Fig. 3.32).
Ribosomes have specific attachment sites that allow tRNA molecules and mRNA to be in the proper close contact that they require to synthesize proteins. Two of these sites are tRNA pockets called the P site and the A site. The other sites are mRNA grooves. There is also a site where an enzyme called peptidyl transferase works to form bonds between adjacent amino acids.
In eukaryotic cells ribosomes have two parts (subunit)- 60 S and 40 S. The 60 S subunit contains the 28 S rRNA, the 5.8 S rRNA, the 5 S rRNA, and around 45 to 50 different proteins. The 40 S subunit contains the 18 S rRNA and around 30 different proteins. The final total size of the completed ribosome is around 80 S where half of the mass is consisted of proteins.
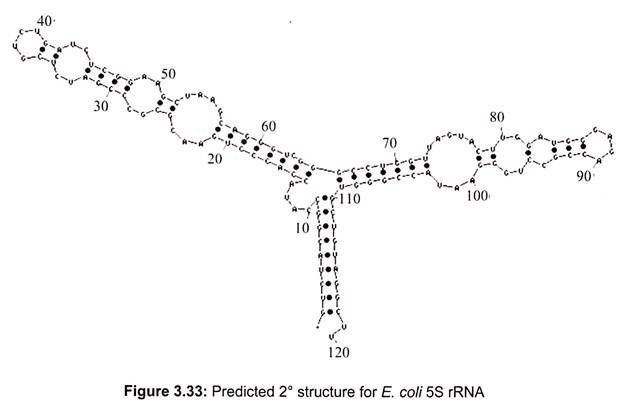
In prokaryotes, a small 30S ribosomal subunit contains the 16S rRNA, while the large 50S ribosomal subunit contains two rRNA species (the 5S and 23S rRNAs). The bacterial 16S, 23S, and 5S rRNA (Fig. 3.33) genes are typically organized as a co-transcribed operon. Archaea contains either a single rDNA operon or multiple copies of the operon. The 3′ end of the 16S rRNA (in a ribosome) binds to a sequence on the 5′ end of mRNA called the Shine-Dalgarno sequence.
(d) Heterogenous Nuclear RNA – hnRNA:
Heterogenous nuclear RNA is defined as all nuclear RNA, excluding rRNA having a sedimentation coefficient greater than 8S (Weinberg, 1973). Eukaryote cells synthesize heterogenous nuclear RNA (hnRNA) which has been considered to be the precursor of mRNA. Only some hnRNA, however, serves as precursor of mRNA. Most of the hnRNA synthesized in eukaryote cells is rapidly degraded without becoming mRNA. Ribosomal RNA from mammalian cells has a high G + C content, while the G+C content of hnRNA is considered quite low. About 20-40% of hnRNA molecules are polyadenylated in mammlian cells.
(e) Small Nuclear RNA (snRNA):
snRNA consists of short RNA transcripts of 100-300 bp, it associates with proteins to form small nuclear ribonucleoprotein particles (snRNPs) which participate in RNA processing. Small nuclear RNA (snRNA) is the name used to refer to a number of small RNA molecules found in the nucleus. These RNA molecules are important in a number of processes including RNA splicing (removal of the introns from hnRNA) and maintenance of the telomeres, or chromosome ends. They are always found associated with specific proteins and the complexes are referred to as small nuclear ribonucleoproteins (SNRNP) or sometimes as snurps.
(f) Small Nucleolar RNA (snoRNA):
As the name suggests, these small (60-300 nts) RNAs are found in the nucleolus where they are responsible for several functions: Some participate in making ribosomes by helping to cut up the large RNA precursor of the 28S, 18S, and 5.8S molecules. Others chemically modify many of the nucleotides in rRNA, tRNA, and snRNA molecules, e.g., by adding methyl groups to ribose. One snoRNA serves as the template for the synthesis of telomeres. In vertebrates, the snoRNAs are made up of introns which are removed during RNA processing.
(g) Micro RNAs (miRNAs):
They are found in all animals (humans have more than 600 miRNA genes) and plants but not in fungi. It contains 19-25 nucleotides. They may be expressed in only certain cell types and at only certain times in the differentiation of a particular cell type. In repression of mRNA translation probably several miRNAs bind simultaneously in the 3′-UTR region.
5. Essay on the Architects behind the Discovery of DNA:
A-primary technique for structural analysis of biological molecule is X-ray crystallography. The wavelength of X-rays is about the same as the space between the atoms in crystalline matter. Deflected X-rays can give an image pattern on a photographic plate, whose angles when analyzed mathematically can lead to the details of each atoms arranged with respect to the other atoms.
Rosalind Elise Franklin was born in 1920 in London and attended St. Paul’s Girls School where she excelled in science, mathematics, and athletics. In 1938, she was awarded a scholarship in physics and chemistry to attend Cambridge University where she undertook studies in X-ray crystallography. After earning her Ph.D. and publishing seminal papers on coal she took a job offer in one of the best labs in Paris. She was a good experimenter, perfected her X-ray techniques, and published, spoke at conferences, and was well liked by her peers. It has been reported that she was a fashion-minded lady of Paris wearing Dior and socializing as a chef for her friends. After 4 years in Paris she decided at 30 years of age to return to London. She was hired by J.T. Randall, Director of King’s College biophysics labs, to create an X-ray unit and work on DNA.
Maurice Wilkins, the assumed overseer of the King’s College lab, had in 1951 taken the first X-ray pictures of DNA that led him to suggest the DNA structure might be a helix (similar to just announced Linus Pauling alpha helical structure of proteins). The atmosphere at King’s was akin to an old boy’s club (the lunch room was from men only) which lead to conflict. In addition, Randall did not clearly delineate a chain of command, and though he had hired Franklin as director of the X-ray lab, Wilkins, who was away when Franklin was hired, believed himself to be in charge. Rudolf Signer, a Swiss chemist had isolated some quality calf thymus DNA, which he gave in a “jelly jar“ to Maurice Wilkins at a scientific meeting sometime in 1951. At the time this was the… “best sample of DNA in the world”. Franklin was given Signer’s DNA by the King’s College biophysics lab director, J.T. Randall.
Watson and Crick pursued model building, using balls and sticks. Their first model was a triple helix with the bases pointed outward. However, chemically it wouldn’t hold itself together, thus they knew it was incorrect.
At the age of 38 (1958) Rosalind Franklin died of ovarian cancer, probably due to constant exposure to X-rays. In 1962 Francis Crick, James Watson, and Maurice Wilkins shared the Nobel Prize.
6. Essay on the Summary of Nucleic Acid:
Biochemistry aims to explain biological form and function in chemical terms. The major biological phenomena have been revealed by purifying individual chemical component, such as a protein, from a living organism and its structural and chemical characterization.
Proteins are long polymers of amino acids, constitute one of the largest fractions of cells. Some proteins have catalytic activity and function as enzymes; others serve as structural elements, signal receptors, or transporters that carry specific substances into or out of cells. The nucleic acids, DNA and RNA, are polymers of nucleotides. They store and transmit genetic information, and some RNA molecules have structural and catalytic roles in molecular complexes.
The polysaccharides are polymers of simple sugars such as glucose. They have two major functions:
As energy-yielding fuel stores and as extracellular structural elements with specific binding sites for particular proteins. Shorter polymers of sugars (oligosaccharides) attached to proteins or lipids at the cell surface serve as specific cellular signals.
The lipids, greasy or oily hydrocarbon derivatives, serve as structural components of membranes, energy-rich fuel stores, pigments, and intracellular signals. In proteins, nucleotides, polysaccharides, and lipids, the number of monomeric subunits is very large- molecular weights in the range of 5,000 to more than l million for proteins, up to several billion for nucleic acids, and in the millions for polysaccharides such as starch.
The covalent bonds and functional groups of a biomolecule are, of course, central to its function, but so also is the arrangement of the constituent atoms of a molecule in three- dimensional space, its stereochemistry. A carbon-containing compound commonly exists as stereoisomers, molecules with the same chemical bonds but different stereochemistry that is, different configuration and the fixed spatial arrangement of atoms. Interactions between bio- molecules are invariably stereospecific, requiring specific stereochemistry in the interacting molecules. A nearly universal set of several hundred small molecules is found in living cells; the inter-conversions of these molecules in the central metabolic pathways have been conserved in evolution.
The central issue in bioenergetics (the study of energy transformations in living systems) is the means by which energy from fuel metabolism or light capture is coupled to energy- requiring reactions of the cell. All biological macromolecules are much less thermodynamically stable than their monomeric subunits, yet they are kinetically stable: their uncatalyzed breakdown occurs so slowly that, on a time scale that matters for the organism, these molecules are stable.
Virtually every chemical reaction in a cell occurs at a significant rate only because of the presence of enzymes—biocatalysts that, like all other catalysts, greatly enhance the rate of specific chemical reactions without being consumed up in the process. Cellular catalysts are, with a few exceptions, proteins. A further contribution to catalysis occurs when two or more reactants bind to the enzyme’s surface close to each other and with stereo- specific orientations that favor the reaction. This increases by orders of magnitude the probability of productive collisions between reactants.
The thousands of enzyme-catalyzed chemical reactions in cells are functionally organized into many sequences of consecutive reactions, called pathways, in which the product of one reaction becomes the reactant in the next. Some pathways degrade organic nutrients into simple end products in order to extract chemical energy and convert it into a form useful to the cell; together these degradative, free -energy-yielding reactions are designated as catabolism.
Other pathways start with small precursor molecules and convert them to progressively larger and more complex molecules, including proteins and nucleic acids. Such synthetic pathways, which invariably require the input of energy, are collectively designated as anabolism. The overall network of enzyme- catalyzed pathways constitutes cellular metabolism. ATP is the major connecting link (the shared intermediate) between the catabolic and anabolic components of this network.
All cells are bounded by a plasma membrane; have a cytosol containing metabolites, coenzymes, inorganic ions and enzymes; and have a set of genes contained within a nucleoid (prokaryotes) or nucleus (eukaryotes). Bacterial cells contain cytosol, a nucleoid and plasmids. Eukaryotic cells have a nucleus and are multicompartmented, segregating certain processes in specific organelles, which can be separated.