With some initial difficulties, the fruit fly Drosophila melanogaster has emerged as an excellent model organism, for the study of development. The zygote develops from the egg. Maternal mRNAs and proteins are the first expressed in the embryo.
Drosophila: The organism that unravelled mysteries of genetics
These substances first determine the broad pattern of the embryo. Then, through, signal pathways involving numerous specific transcription factors, they initiate a cascade of gene expression that eventually determines the fate of each cell.
Initially, Drosophila was found to be a difficult organism to study its embryology. Jack Schutz and others (1930’s) attempted to relate the genetics of Drosophila to its development, but, the fly embryos proved too complex to manipulate experimentally and nor transparent enough to observe.
Christiane Nusslein-Volhard (1988) worked on origin and polarity in Drosophila. She also worked on bicoid protein. Edward B. Lewis, Nusslein-Volhard and Eric Wieschans awarded 1995 Nobel Prize in Medicine and Physiology for genetic control of embryonic development.
In this article, we will concentrate on two overall patterns of development: the formation of the basic body plan (anterior-posterior and dorsal-ventral polarity, which results in a segmented embryo that has a front, back, top and bottom) and the determination of gene expression within segments.
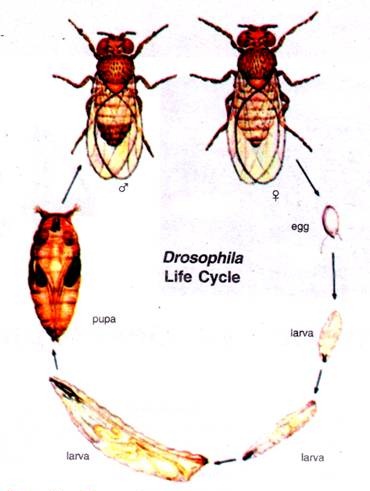
Beginning with the fertilized egg, Drosophila passes through a pre-adult period of development with five distinct phases; the embryo, three larval stages and the pupal stage. The adult fly emerges from the pupal case about 10 days after fertilization.
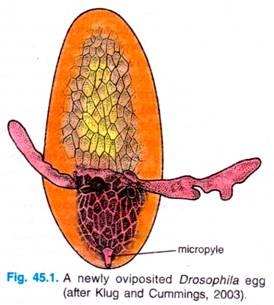
Externally, the Drosophila egg has a number of structures that characterise the anterior, posterior, dorsal and ventral regions. The anterior end of the egg contains the micropyle, a specialised conical structure for the entrance of sperm into the egg, while the posterior end is rounded and marked by a series of aeropyles (openings that allow gas exchange during development).
The dorsal side of the egg is flattened and contains the chorionic appendages, while the ventral side is curved. Internally, the egg cytoplasm is organised into a series of maternally derived molecular gradients. These gradients play a key role in establishing the development fate of nuclei that migrate into specific regions of the embryo.
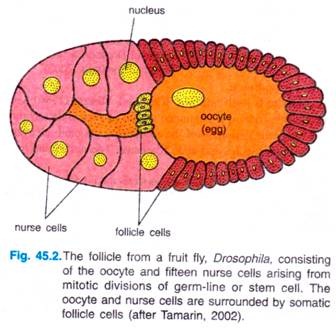
The development of oocyte of Drosophila begins in a germarium of a follicle, with stem cells at one end. A germ-line cell or stem cell undergoes four mitotic divisions to give 16 cells with cytoplasmic bridges between each other.
One of 16 cells will become the oocyte, the other 15 will develop into nurse cells, which produce large quantities of proteins and RNAs that are imported into the egg (oocyte) through the cytoplasmic bridges. Somatic ovarian cells form a sheath of follicle cells around the nurse cells and oocyte to form the egg chamber, and they have key role in patterning the egg’s axes.
There are various types of follicle cells which express different genes and have different effects on the oocyte. Follicle cells also secrete the materials of the vitelline envelope and egg shell that surround the mature egg.
Cleavage:
During cleavages of fertilized egg of most animals occurs the specification of early embryonic cells by their acquisition of different cytoplasmic determinant that had been stored in the oocyte. The cell (plasma) membranes establish the region of cytoplasm incorporated into each new blastomere, and it is thought that the morphogenetic determinants then direct differential gene expression in these blastomeres.
However, during Drosophila development blastomeres do not form until after the thirteenth nuclear division. Prior to this time, all the nuclei share a common cytoplasm and material can diffuse throughout the syncitial cytoplasm of embryo.
In these embryos, the specification of cell types along anterior-posterior and dorsal-ventral axes is accomplished by interactions of cytoplasmic materials within the single, multinucleated cell. Moreover, the initiation of the anterior-posterior and dorsal-ventral differences is fusion of parental gamete nuclei, controlled by the position of the egg within mother’s ovary.
Whereas the sperm entry site may fix the axes in ascidians and nematodes, the fly’s anterior-posterior and dorsal-ventral axes are specified by interactions between the egg and its surrounding follicle cells.
Drosophila and most eggs undergo superficial produce multinucleated syncytium, cleavage, wherein a large mass of centrally located yolk confines cleavage to the cytoplasmic rim of the egg. One of the interesting features of this cleavage type is that cells do not form until after the nuclei are divided.
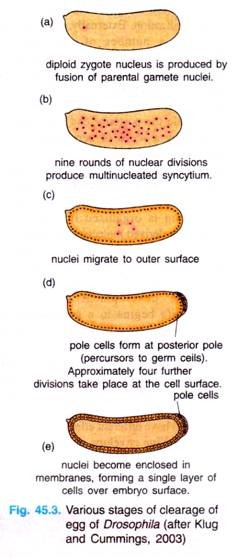
The zygote nucleus undergoes several mitotic divisions within the central portion of the egg. In Drosophila, 256 nuclei are produced by a series of eight nuclear divisions averaging 8 minutes each. The nuclei then migrate to the periphery of the egg, where the mitosis continue, albeit at a progressively slower rate.
During the ninth division cycle, about five nuclei reach the surface of the posterior pole cells form at posterior pole of the embryo. These nuclei become enclosed by cell membranes and generate the pole cells that contain so-called germplasm and give rise to the gametes of the adult.
Most of other nuclei arrive at the periphery of the embryo at cycle 10 (at tenth cell cycle, there are 512 nuclei and stage occurs 2 hours after fertilization) and then undergoes four more divisions at progressively slower rates.
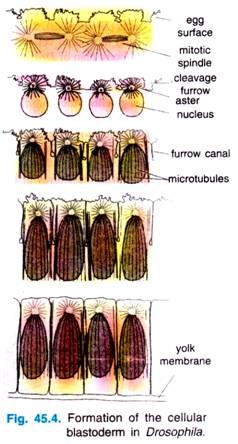
During these stages of nuclear division, the membranes, forming a single layer of embryo is called a syncytial blastoderm, meaning that all the cleavage nuclei are contained within a common cytoplasm. No cell membranes exist other than that of the egg itself.
Although the nuclei divide within a common cytoplasm, this does not mean that the cytoplasm is itself uniform. Karr and Albert’s (1986) have shown that each nucleus within the syncytial blastoderm is contained within its own little territory of cytoskeleton proteins.
When the nuclei reach the periphery of the egg during the tenth cleavage cycle, each nucleus becomes surrounded by microtubules and actin filaments. The nuclei and their associated cytoplasmic islands are called synergids.
Following cycle 13, the oocyte plasma membrane folds inward between the nuclei, eventually partitioning off each somatic nucleus into a single cell (Fig. 45.3). This process creates the cellular blastoderm, in which all the cells are arranged in a single-layer jacket around the yolky core of the egg. In Drosophila, the cellular blastoderm consists of approximately 6000 cells and is formed within 4 hours of fertilization.
The Midblastula Transition:
After the nuclei reach the periphery, the time required to complete each of the next four divisions become progressively longer. Thus, while 1-10 cleavage cycles are each 8 minutes long, cycle 13, the last cycle in the syncytial blastoderm, takes 25 minutes to complete. Cycle 14, in which the Drosophila embryo forms cells (i.e., after 13 divisions), is asynchronous.
Some groups of cells complete this cycle in 75 minutes, whereas other groups of cells take 175 minutes. Transcription from the nuclei (which begins around the eleventh cycle) is greatly enhanced at this stage. This slow-down of nuclear division and the simultaneous increase in RNA transcription is often referred to as the midblastula transition.
Gastrulation and Larva Formation:
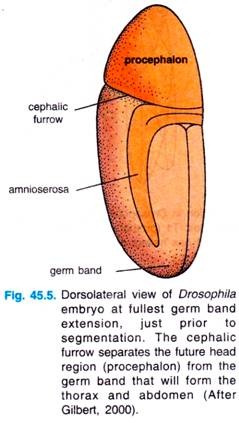
Gastrulation begins at the time of midblastula transition. The first movements segregate the presumptive mesoderm, endoderm and ectoderm. The presumptive mesoderm — about 1000 cells constituting the ventral midline of the embryo — folds inward to produce the ventral furrow (Fig. 45.5).
This furrow eventually pinches off from the surface to become a ventral tube within the embryo. It then flattens to form a layer of mesodermal tissue beneath the ventral ectoderm.
The prospective endoderm invaginates as two pockets at the anterior and posterior ends of the ventral furrow. The pole cells are internalized along with the endoderm. At this time, the embryo bends to form cephalic furrow.
The ectodermal cells on the surface and the mesoderm undergo convergence and extension, migrating toward the ventral midline to form the germ band. The germ band is a collection of cells along the ventral midline that includes all the cells that will form the trunk of the embryo.
The germ band extends posteriorly and perhaps because of the egg case, wrap around the top (dorsal) surface of the embryo (Fig. 45.5). Thus, at the end of germ band formation, the cells destined to form the most posterior larval structures are located immediately behind the future head region.
At this time the body segments begin to appear, dividing the ectoderm and mesoderm. Lastly, the germ band, retracts, placing the presumptive Posterior segments into the posterior tip of the embryo.
While the germ band is in its extended position, extension, just prior to several key morphogentic processes occur: segmentation. The cephalic organogenesis, segmentation and the segregation of furrow separates the future head the imaginal discs.
The imaginal discs are those cells which are set aside to produce the adult structures. In addition, the nervous system forms from two regions of ventral ectoderm. Neuroblasts differentiate from this neurogenic ectoderm within each segment (also from the non-segmented region of the head ectoderm). Therefore, in insects (Drosophila), the nervous system is located ventrally, rather than being derived from a dorsal neural tube as in vertebrates.
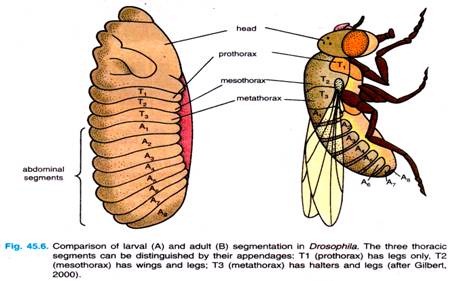
The general body plan of Drosophila is the same in the embryo, the larva and the adult, each of which has a distinct head end and a distinct tail end, between which are repeating segmented units (Fig. 45.6). Three of these segments form the thorax, while another eight segments form the abdomen.
Each segment of the adult fly has its own identity. The first thoracic segment, for example has only legs; the second thoracic segment has legs and wings; and the third thoracic segment has legs and halters (balancers). Thoracic and abdominal segments can also be distinguished from each other by differences in cuticle.
The embryo also has an anterior tip, the acron, that will give rise to structures at the very head end — eyes and antennae, and a posterior tip, called the telson, that will give rise to the internal structures at the very posterior end of the fly. Acron and telson, both, represent non-segmented parts of the embryo.
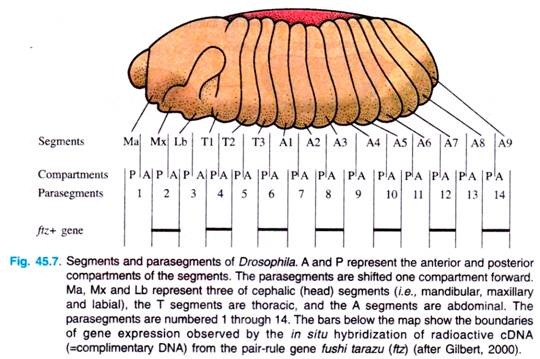
At about six hours of development, furrows become visible in the embryo, depicting segments. The first segments visible are called parasegments; they are 14 in number. Parasegments are regions of the embryo that are separated by mesodermal thickenings and ectodermal grooves.
They do not give rise to the later segments (i.e., segments of larva and adult) in a simple fashion: each later segment is made up of the anterior end of one parasegment and the posterior end of the next (Fig. 45.7).