The following points highlight the thirteen main factors that help in determining the water quality. The factors are: 1. Temperature 2. Specific Conductance 3. pH 4. Dissolved Oxygen 5. Total Organic Carbon 6. Hardness 7. Alkalinity 8. Ammonia 9. Phosphate 10. Fecal Coliform Bacteria 11. Turbidity 12. Total Suspended Solids 13. Total Dissolved Solids.
Factor # 1. Temperature:
Temperature of water is a very important factor for aquatic life. It controls the rate of metabolic and reproductive activities, and determines which fish species can survive. Temperature also affects the concentration of dissolved oxygen and can influence the activity of bacteria and toxic chemicals in water.
Factors Affecting Temperature:
(i) Riparian Vegetation:
Riparian vegetation, or trees and plants growing along the banks of a river or creek, provide shade, preventing the sun from heating up the water. If the sun shines directly on water, the water can warm up very quickly, and to very high temperatures.
(ii) Flow Rate:
During dry seasons, there is less water in a river or creek, and it flows more slowly. This allows the water to warm up more quickly, and to warmer temperatures.
(iii) Paved Surfaces:
As business and housing developments are built, previously open lands are covered with buildings and pavement. This covered area is called “impermeable surface.” Less rainwater is soaked into the ground, and more of it runs over land into streams during storms. This run-off also moves faster into the stream than would natural run-off because it travels through straight concrete or plastic storm drain pipes.
This increased volume and velocity of run-off scours the stream channel and widens it. During dry weather between storms, the channels have a very shallow flow. These wide, shallow streams heat up much more quickly than do more natural narrow, deep ones.
Additionally, black surfaces, like many streets and parking lots, absorb heat, and rainwater moving over these surfaces during storm events becomes warmer.
(iv) Industrial Discharge:
Some industries use water as a coolant during processing. This water is sometimes discharged to a stream or lake. When this water is discharged to a creek, it is much warmer than the water in the creek, and the temperature of the creek becomes higher. This phenomenon is called thermal pollution. This can cause the period of ice cover on the water to be shortened, and can increase the metabolic rate of plants and animals, producing an increase in oxygen demand.
(v) Sewage Outflow:
Water discharged from treatment plants is warm because of the wastewater entering the plant (coming from our homes and businesses) is warm. This is another form of thermal pollution.
Temperature preferences among aquatic species vary widely, but all species tolerate slow, seasonal changes better than rapid changes.
Factor # 2. Specific Conductance (SC):
Specific Conductance (SC) is a measure of how well water can conduct an electrical current. Conductivity increases with increasing amount and mobility of ions. These ions, which come from the breakdown of compounds, conduct electricity because they are negatively or positively charged when dissolved in water. Therefore, SC is an indirect measure of the presence of dissolved solids such as chloride, nitrate, sulfate, phosphate, sodium, magnesium, calcium, and iron, and can be used as an indicator of water pollution.
Measurement of SC:
Specific conductance measures how well water can conduct an electrical current for a unit length and unit cross-section at a certain temperature. More specifically, it is defined as the reciprocal (opposite) of the resistance in ohms measured between opposite faces of a centimetre cube of an aqueous solution at a specified temperature”. That is, conductance = 1/resistance
Specific conductance is measured using a sensor which measures resistance. Resistance means how well something can resist an electrical current, and is reported in ohms. The unit of conductance was originally ohm spelled backwards – “mho.” More recently, however, the name “siemen” has been used to match the term used by the International System of Units.
So both “mho” and “siemen” are sometimes seen in water quality reports. One siemen is equal to one mho. Because SC in natural waters is usually much less than 1 siemen/cm, SC is usually reported in microsiemens (1/1,000,000 siemen) per centimetre, or µS/cm. SC is affected by temperature, so for consistency SC values are converted to what they would be at room temperature (25 °C).
Factors Affecting SC:
(i) Geology and Soil in the Watershed:
Some rock and soil release ions very easily when water flows over them; for example, if acidic water flows over rocks containing calcite (CaCO3), such as calcareous shales, calcium (Ca2+) and carbonate (CO32-) ions will dissolve into the water. Therefore, SC will increase. However, some rocks, such as quartz (SiO2), are very resistant, and don’t dissolve easily when water flows over them (and even if SiO2 does dissolve, it is not conductive). SC of waters draining areas where the geology only consists of quartz or other resistant rocks will be low (unless other factors are involved).
(ii) Acid Mine Drainage:
Drainage from operating and abandoned mine sites can contribute iron, sulfate, copper, cadmium, arsenic, and/or other constituents if minerals containing these constituents are present and are exposed to air and water. This increase in ions will increase the SC.
(iii) Agricultural Run-Off:
Run-off from farms can contain fertilizers, which contain phosphate and nitrate. In addition, irrigation return flow can leach salts from the soil.
(iv) Road Run-Off:
Run-off from roads can contain leaked automobile fluids and salts from chemicals used in road de-icing, such as sodium chloride (NaCl) and magnesium chloride (MgCl2).
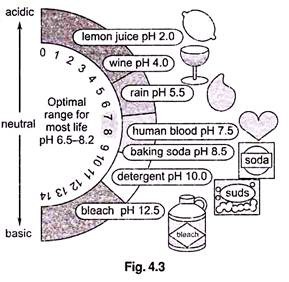
Factor # 3. pH:
pH represents the effective concentration (activity) of hydrogen ions (H+) in water. This concentration could be expressed in the same kind of units as other dissolved species, but H concentrations are much smaller than other species in most waters. The activity of hydrogen ions can be expressed most conveniently in logarithmic units. pH is defined as the negative logarithm of the activity of H+ ions–
pH = – log [H+]
where [H+] is the concentration of H ions in moles per litre (a mole is a unit of measurement, equal to 6.022 × 1023 atoms). Because H+ ions associate with water molecules to form hydronium (H3O+) ions, pH is often expressed in terms of the concentration of hydronium ions. In pure water at 22 °C (72 °F), H3O+ and hydroxyl (OH–) ions exist in equal quantities; the concentration of each is 1.0 × 10-7 moles per litre (mol/L). Therefore, pH of pure water = – log (1.0 x 10-7) = – (- 7.00) = 7.00.
Because pH is defined as – log [H+], pH decreases as [H+] increases (which will happen if acid is added to the water). Since pH is a log scale based on 10, the pH changes by 1 for every power of 10 change in [H+], A solution of pH 3 has an H+ concentration 10 times that of a solution of pH 4. The pH scale ranges from 0 to 14. However, pH values less than 0 and greater than 14 have been observed in very rare concentrated solutions.
Measurement of pH:
The pH of water can be measured with a pH metre, which is an electronic device with a probe. The probe contains an acidic aqueous solution enclosed by a glass membrane that allows migration of H+ ions. The electrical potential of the glass electrode depends on the difference in [H+] between the reference solution and the solution into which the electrode is dipped. pH can also be measured with pH paper or by adding a reagent (indicator solution) to the water sample and recording the color change.
Factor # 4. Dissolved Oxygen (DO):
Dissolved Oxygen (DO) is found in microscopic bubbles of oxygen that are mixed in the water and occur between water molecules. DO is a very important indicator of a water body’s ability to support aquatic life. Fish “breathe” by absorbing dissolved oxygen through their gills. Oxygen enters the water by absorption directly from the atmosphere or by aquatic plant and algae photosynthesis. Oxygen is removed from the water by respiration and decomposition of organic matter.
Measurement of DO:
Dissolved oxygen can be measured with an electrode and metre or with field test kits. The electronic metre does not measure oxygen directly; rather, it uses electrodes to measure the partial pressure of oxygen in the water, which is converted to oxygen mass weight concentration.
The field test kits (such as a drop bottle, a microburet, or a digital titrator) involve adding a solution of known strength to a treated sample of water from the stream. The amount of solution required to change the color of the sample reflects the concentration of DO in the sample. The amount of oxygen dissolved in water is expressed as a concentration, in milligrams per litre (mg/1) of water.
Dissolved oxygen levels are also often reported in percent saturation. Temperature affects DO concentrations, and calculating the percent saturation will factor out the effect of temperature.
The “saturation level” is the maximum concentration of dissolved oxygen that would be present in water at a specific temperature, in the absence of other factors. Scientists have determined the saturation DO level for various temperatures. Saturation levels also vary with elevation. Percent saturation is calculated by dividing the measured dissolved oxygen concentration by the saturation level and multiplying by 100.
This equation is shown as:
% Saturation = (DO/Saturation Level) × 100.
Factor # 5. Total Organic Carbon (TOC):
Organic matter plays a major role in aquatic systems. It affects biogeochemical processes, nutrient cycling, biological availability, chemical transport and interactions. It also has direct implications in the planning of wastewater treatment and drinking water treatment. Organic matter content is typically measured as total organic carbon and dissolved organic carbon, which are essential components of the carbon cycle. Organic matter in water consists of thousands of components, including macroscopic particles, colloids, dissolved macromolecules, and specific compounds.
Measurement of TOC:
The TOC in Boulder Creek samples is analyzed at the U.S. Geological Survey on a Sievers Model 800 Carbon Analyzer. TOC concentration is not directly measured; the Analyzer measures total carbon (TC) and total inorganic carbon (TIC) and subtracts TIC from TC to obtain TOC. An oxidizer and an acid are added to the sample.
The acid reacts with bicarbonate and carbonate ions present in the sample to release carbon dioxide (CO2). The CO2 released from bicarbonate and carbonate ions represents the TIC in the sample.
The sample is then subjected to ultra-violet (UV) radiation, which reacts with the oxidant and breaks down all remaining carbon bonds in the sample to release CO2. The CO2 released from both the acid reaction and the UV radiation represents all the carbon (TC) released from the sample. TOC is then obtained by subtracting TIC from TC.
The TOC of a water body is affected by several factors, including:
i. Vegetation,
ii. Climate, and
iii. Treated Sewage.
Factor # 6. Hardness:
Hardness is measure of polyvalent cations (ions with a charge greater than +1) in water. Hardness generally represents the concentration of calcium (Ca2+) and magnesium (Mg2+) ions, because these are the most common polyvalent cations. Other ions, such as iron (Fe2+) and manganese (Mn2+), may also contribute to the hardness of water, but are generally present in much lower concentrations. Waters with high hardness values are referred to as “hard,” while those with low hardness values are “soft”. Affects the amount of soap that is needed to produce foam or lather.
Hard water requires more soap, because the calcium and magnesium ions form complexes with soap, preventing the soap from sudsing. Hard water can also leave a film on hair, fabrics, and glassware. Hardness of the water is very important in industrial uses, because it forms scale in heat exchange equipment, boilers, and pipe lines. Some hardness is needed in plumbing systems to prevent corrosion of pipes.
Hardness mitigates metals toxicity, because Ca2+ and Mg2+ help keep fish from absorbing metals such as lead, arsenic, and cadmium into their bloodstream through their gills. The greater the hardness, the harder it is for toxic metals to be absorbed through the gills.
Measurement of Hardness:
Hardness is generally measured by titration. A buffer and a color indicator are added to a volume of water. An acid (the titrant) is then added to the water, and it reacts with the Ca2+ and Mg2+ in the water. The volume of acid required to change the color of the sample reflects the Ca2+ and concentration of the sample. The more acid needed, the more Ca2+ and Mg2+ in the sample. Hardness is generally expressed in units of milligrams per liter (mg/1) or parts per million (ppm) of CaCO3 (calcium carbonate). It can also be expressed in “grains per gallon” (gpg); one gpg equals approximately 17 mg/L.
Hardness can also be calculated from measurements of calcium and magnesium using the following formula:
Hardness, mg equivalent/L CaCO3 = ([Ca, mg/l]*2.497) + ([Mg, mg/l]*4.116).
Factors Affecting Hardness:
(i) Geology:
Soft waters are mainly derived from the drainage of igneous rocks, because these rocks don’t weather very easily and so don’t release many cations. Hard water is often derived from the drainage of calcareous (calcite-rich) sediments, because calcite (CaCO3) dissolves, releasing the calcium. Calcium, magnesium, and other polyvalent cations such as iron and manganese may be added to a natural water system as it passes through soil and rock containing large amounts of these elements in mineral deposits.
(ii) Mining:
Drainage from operating and abandoned mine sites can contribute calcium, magnesium, iron, manganese, and other ions if minerals containing these constituents are present and are exposed to air and water. This can increase the hardness of a stream.
(iii) Industrial Discharge:
Some industrial processes may also produce significant amounts of calcium and manganese that are later discharged into streams.
(iv) Sewage Outflow:
The effluent from Wastewater Treatment Plants (WWTPs) can add hardness to a stream. The wastewater from our houses contains calcium, magnesium, and other cations from the cleaning agents, food residue, and human waste that we put down our drains. Most of these cations are removed from the water at the WWTP before being discharged to the stream, but treatment can’t eliminate everything.
Factor # 7. Alkalinity:
Alkalinity is a measure of the buffering capacity of water, or the capacity of bases to neutralize acids. Measuring alkalinity is important in determining a stream’s ability to neutralize acidic pollution from rainfall or wastewater. Alkalinity does not refer to pH, but instead refers to the ability of water to resist change in pH.
The presence of buffering materials help neutralize acids as they are added to the water. These buffering materials are primarily the bases bicarbonate (HCO3–), and carbonate (CO32-), and occasionally hydroxide (OH–), borates, silicates, phosphates, ammonium, sulfides, and organic ligands.
Waters with low alkalinity are very susceptible to changes in pH. Waters with high alkalinity are able to resist major shifts in pH. As increasing amounts of acid are added to a water body, the pH of the water decreases, and the buffering capacity of the water is consumed.
If natural buffering materials are present, pH will drop slowly to around 6; then a rapid pH drop occurs as the bicarbonate buffering capacity (CO32- and HCO3–) is used up. At pH 5.5, only very weak buffering ability remains, and the pH drops further with additional acid. A solution having a pH below 4.5 contains no alkalinity, because there are no CO32- or HCO3– ions left.
Alkalinity not only helps regulate the pH of a water body, but also the metal content. Bicarbonate and carbonate ions in water can remove toxic metals (such as lead, arsenic, and cadmium) by precipitating the metals out of solution.
Measurement of Alkalinity:
Alkalinity is measured by titration. An acid of known strength (the titrant) is added to a volume of a treated sample of water. The volume of acid required to bring the sample to a specific pH level reflects the alkalinity of the sample. The pH end point is indicated by a color change. Alkalinity is expressed in units of milligrams per litre (mg/1) of CaCO3 (calcium carbonate).
Factors Affecting Alkalinity:
(i) Geology and Soils:
Carbonates are added to a water system if the water passes through soil and rock that contain carbonate minerals, such as calcite (CaCO3). Where limestone and sedimentary rocks and carbonate-rich soils are predominant, (such as the eastern part of the Boulder Creek watershed) waters will often have high alkalinity. Where igneous rocks (such as granite) and carbonate-poor soils are predominant (such as the western part of the Boulder Creek watershed) waters will have low alkalinity.
(ii) Changes in pH:
Because alkalinity and pH are so closely related, changes in pH can also affect alkalinity, especially in a poorly buffered stream. See the section on pH for more information on factors affecting pH.
(iii) Sewage Outflow:
The effluent from Wastewater Treatment Plants (WWTPs) can add alkalinity to a stream. The wastewater from our houses contains carbonate and bicarbonate from the cleaning agents and food residue that we put down our drains.
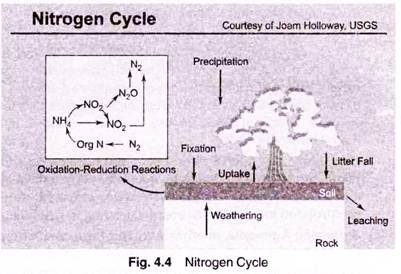
Factor # 8. Ammonia—The Nitrogen Cycle:
Nitrogen is recycled continually by plants and animals. This recycling of nitrogen through the environment is called the “nitrogen cycle.”
Most organisms (including humans) can’t use nitrogen in the gaseous form N2 for their nutrition, so they are dependent on other organisms to convert nitrogen gas to nitrate, ammonia, or amino acids. “Fixation” is the conversion of gaseous nitrogen to ammonia or nitrate.
The most common kind of fixation is “biological fixation” which is carried out by a variety of organisms, including blue-green algae, the soil bacteria Azobacter, and the association of legume plants and the bacteria Rhizobium. Additionally, nitrogen can be fixed by some inorganic processes. For example, “high-energy fixation” occurs in the atmosphere as a result of lightning, cosmic radiation, and meteorite trails. Atmospheric nitrogen and oxygen combine to form nitrous oxides (NOx), which fall to the earth as nitrate.
When plants and animals die, proteins (which contain organic nitrogen) are broken down by bacteria to form ammonia (NH3). This process is called “ammonification.” Ammonia is then broken down by other bacteria (Nitrosomonas) to form nitrite (NO2), which is then broken down by another type of bacteria (Nitrobacter) to form nitrate (NO3).
This conversion of ammonia to nitrate and nitrite is called “nitrification.” Nitrates can then be used by plants in order to grow. Completing the nitrogen cycle, nitrates are reduced to gaseous nitrogen by the process of “denitrification.” This process is performed by organisms such as fungi and the bacteria Pseudomonas. These organisms breakdown nitrates to obtain oxygen.
Factors Affecting Nitrate + Nitrite Concentrations:
(i) Wastewater and Septic System Effluent:
Human waste is significant contributor of nitrogen to water. Ammonia, nitrite, and nitrate are decomposition products from urea and protein, which are in human waste. Ammonia is an ingredient in many household cleaning products and is sometimes used to remove carbonate from hard water. Therefore, these nitrogen species go down the drains in our houses and businesses, and can enter streams from Wastewater Treatment Plant (WWTP) effluent, illegal sanitary sewer connections, and poorly functioning septic systems.
Nutrients in sewage effluent have been among the primary targets of pollution-control legislation, beginning with the Clean Water Act in 1972. Organic forms of nitrogen have largely been controlled by upgrading treatment plants, and advanced treatment processes have been used to decrease ammonia discharge. However, these processes result in an increase in nitrate discharge, so the total nitrogen discharge does not change. Therefore, concerns about fish toxicity have decreased, but the potential for eutrophication has not changed.
(ii) Fertilizer Runoff:
Fertilizer is a major influence on nitrogen concentrations in the environment. Commercial nitrogen fertilizers are applied either as ammonia or nitrate, but ammonia is rapidly converted to nitrate in the soil. Animal manure is also used as a nitrogen fertilizer in some areas.
Organic nitrogen and urea in the manure are converted to ammonia and, ultimately, to nitrate in the soil. Nitrate that is not used by plants washes from farmlands and residential and commercial lawns into storm drains and nearby streams, or seeps into groundwater.
(iii) Animal Waste:
A significant amount of nitrogen is released in the wastes produced by animals. This can be a serious problem in waters near cattle feedlots, hog farms, dairies, and barnyards. Ducks and geese contribute a heavy load of nitrogen if they are present in large numbers. Excretions of aquatic organisms are very rich in ammonia, a decay product of animal proteins, but the amount of nitrogen they add to waters is usually small. Through the process of nitrification, ammonia is oxidized to nitrite and then to nitrate in water.
(iv) Fossil Fuels:
The burning of fossil fuels such as gasoline and coal in cars, trucks, and power plants produces many by products. Coal and petroleum generally contain about 1 percent nitrogen. Part of the nitrogen is converted to the gas nitric oxide (NO) during the burning of the fuel. Nitric oxide is converted by sunlight and photochemical processes in air to nitrogen oxide gases (NO and NO2, which are commonly referred together as NOx), which are a major component of smog. Nitrogen oxide gases are a major contributor to acid rain.
(v) Industrial Discharge:
Many industries use nitrogen during processing. Nitrite is sometimes used as a corrosion inhibitor in industrial process water. Ammonia is used in the production of nitric acid, urea and other nitrogen compounds, and in the production of ice and in refrigerating plants. Ammonia is also used in cleaning supplies and to remove carbonate from hard water. Water from industries is usually discharged to a Wastewater treatment plant (WWTP), and may end up in a downstream water body if not completely removed in the WWTP.
Factor # 9. Phosphorus:
Phosphorus is a nutrient required by all organisms for the basic processes of life. Phosphorus is a natural element found in rocks, soils and organic material. Phosphorus clings tightly to soil particles and is used by plants, so its concentrations in clean waters is generally very low. However, phosphorus is used extensively in fertilizer and other chemicals, so it can be found in higher concentrations in areas of human activity. Many seemingly harmless activities added together can cause phosphorus overloads.
Phosphorus exists in water in either a particulate phase or a dissolved phase. Particulate matter includes living and dead plankton, precipitates of phosphorus, phosphorus adsorbed to particulates, and amorphous phosphorus. The dissolved phase includes inorganic phosphorus and organic phosphorus. Phosphorus in natural waters is usually found in the form of phosphates (PO4-3). Phosphates can be in inorganic form (including orthophosphates and polyphosphates), or organic form (organically bound phosphates).
(i) Organic phosphate is phosphate that is bound to plant or animal tissue. Organic phosphates are formed primarily by biological processes. They are contributed to sewage by body waste and food residues, and also may be formed from orthophosphates in biological treatment processes or by receiving water biota. Organic phosphates may occur as a result of the breakdown of organic pesticides which contain phosphates. They may exist in solution, as loose fragments, or in the bodies of aquatic organisms.
(ii) Inorganic phosphate is phosphate that is not associated with organic material. Types of inorganic phosphate include orthophosphate and polyphosphates. Orthophosphate is sometimes referred to as “reactive phosphorus.” Orthophosphate is the most stable kind of phosphate, and is the form used by plants. Orthophosphate is produced by natural processes and is found in sewage.
Polyphosphates (also known as metaphosphates or condensed phosphates) are strong complexing agents for some metal ions. Polyphosphates are used for treating boiler waters and in detergents. In water, polyphosphates are unstable and will eventually convert to orthophosphate. Phosphates are not toxic to people or animals unless they are present in very high levels. Digestive problems could occur from extremely high levels of phosphate.
In freshwater lakes and rivers, phosphorus is often found to be the growth-limiting nutrient, because it occurs in the least amount relative to the needs of plants. If excessive amounts of phosphorus and nitrogen are added to the water, algae and aquatic plants can be produced in large quantities. When these algae die, bacteria decompose them, and use up oxygen. This process is called eutrophication. Dissolved oxygen concentrations can drop too low for fish to breathe, leading to fish kills. The loss of oxygen in the bottom waters can free phosphorus previously trapped in the sediments, further increasing the available phosphorus.
Measurement of Phosphorus:
There are several forms of phosphorus which can be measured. Total phosphorus (TP) is a measure of all the forms of phosphorus, dissolved or particulate, that are found in a sample. Soluble reactive phosphorus (SRP) is a measure of orthophosphate, the filterable (soluble, inorganic) fraction of phosphorus, the form directly taken up by plant cells. Both phosphorus and orthophosphate are often measured using a colorimetric method, which means the color of treated sample reflects the concentration of the parameter.
If total phosphorus is being measured, all forms of phosphorus are converted to dissolved orthophosphate with acid, persulfate, and heat. A chemical is then added to the water sample. The darker the color of the sample becomes, the more phosphorus present. This test can be done visually, comparing the treated sample to a set of reference colors. However, it is more accurate to use an electronic colorimeter, which uses a light source and a photodetector to find the concentration based on how much light is absorbed by the sample.
Factors Affecting Phosphorus Concentrations:
(i) Wastewater and Septic System Effluent:
Domestic and industrial sewage are very important sources of phosphorus to surface water. Organic phosphates are formed primarily by biological processes. They are contributed to sewage by body waste and food residues. Phosphorus is essential in metabolism so is always present in animal waste. Orthophosphates and polyphosphates can be contributed by detergents, as discussed below.
(ii) Detergents:
Orthophosphates and certain polyphosphates are major constituents of many commercial cleaning preparations. In the 1950s and 1960s, sodium phosphate was used often as a “builder” in households detergent to increase cleaning power. The extensive use of detergents led to major eutrophication problems, and in the 1960s efforts were made by governments, detergent manufacturers, and consumers to reduce the use of phosphates in detergents.
As a result, phosphorus concentrations in many streams and lakes decreased. This was due to limits on the phosphate content of detergent, and also additional treatment used in waste water treatment plants to remove phosphorus. Many states have a ban on phosphates in detergents.
(iii) Fertilizers:
Fertilizers generally contain phosphorus in the form of orthophosphate. Phosphate is not very mobile in soil; it tends to remain attached to solid particles rather than dissolving in water. However, if too much fertilizer is applied, the phosphates are carried into surface waters with storm runoff and also with melting snow. Soil erosion of fertilized fields and lawns can also carry a considerable amount of particulate phosphate to streams.
(iv) Animal Waste:
Phosphorus is essential in metabolism, so is present in animal waste. Therefore, phosphate runoff can be an issue in waters near cattle feedlots, hog farms, dairies, and barnyards.
(v) Development/Paved Surfaces:
Development can cause soil erosion, which will release phosphorus. If swamps and wetlands are drained for development, phosphorus that was buried can be exposed. During the building phase, and after everything has stabilized, phosphorus concentrations in storm water can increase because natural filters such as trees, shrubs, and puddles have been eliminated.
(vi) Industrial Discharge:
Polyphosphates are often added to water to prevent iron oxides or calcium carbonates from forming. If this water is released to streams or lakes, polyphosphates can enter the water body, and will convert to orthophosphate.
(vii) Phosphate Mining:
Phosphate mining, concentrating, and processing are sources of phosphate to rivers in some areas. The most common phosphorus-containing mineral is apatite (Ca5F(PO4)3). There are no significant sources of phosphate minerals in the Boulder Creek Watershed, so this is not a problem in our area.
(viii) Drinking Water Treatment:
Small amounts of orthophosphates or certain polyphosphates are added to some water supplies during treatment.
(ix) Forest Fires:
Forest fires can cause soil erosion, which will release phosphorus bound to soil particles.
(x) Synthetic Materials:
Organophosphates are commonly used as construction materials, flame retardant and plasticizers. Reduced forms of phosphorus are present in certain synthetic organic chemicals, including some that are used in insecticides.
Eutrophication:
Eutrophication is a process that results from accumulation of nutrients in lakes or other water bodies. Eutrophication is a natural process, but can be greatly accelerated by human activities that increase the rate at which nutrients enter the water.
Algae growth is limited by the available supply of phosphorus or nitrogen, so if excessive amounts of these nutrients are added to the water, algae and aquatic plants can grow in large quantities. When these algae die, they are decomposed by bacteria, which use dissolved oxygen. This process is called “eutrophication.”
Dissolved oxygen concentrations can drop too low for fish to breathe, leading to fish kills. Excessive amounts of algae grow into scum on the water surface, decreasing recreational value and clogging water-intake pipes. Rapid decomposition of dense algae scums with associated organisms can give rise to foul odors.
In freshwater lakes and rivers, phosphorus is often the growth limiting nutrient, because it occurs in the least amount relative to the needs of plants. In estuaries and coastal waters, nitrogen is generally the growth limiting nutrient.
“Eutrophic” waters are characterized by high nutrient concentrations, resulting in high productivity of plant growth. Such waters are often shallow, with algal blooms and periods of oxygen deficiency. Slightly or moderately eutrophic water can support a complex web of plant and animal life. However, such waters are generally undesirable for drinking water and other needs.
Waters with extreme nutrient concentrations are called “hypereutrophic.” “Oligotrophic” waters are characterized by extremely low nutrient concentrations, resulting in moderate plant productivity. Oligotrophic lakes are those low in nutrient materials and consequently poor areas for the development of extensive aquatic plants and animals. Such lakes are often deep, with sandy bottoms and very limited plant growth, but with high dissolved-oxygen levels.
Some scientists have categorized trophic status according to phosphorus concentration. Lakes with phosphorus concentrations below 0.010 mg/L are classified as oligotrophic, phosphorus concentrations between 0.010 and 0.020 mg/L are indicative of mesotrophic lakes, and eutrophic lakes have phosphorus concentrations exceeding 0.020 mg/L.
Factor # 10. Total and Fecal Coliform Bacteria:
The coliform bacteria group consists of several genera of bacteria belonging to the family enterobacteriaceae. These mostly harmless bacteria live in soil, water, and the digestive system of animals. Fecal coliform bacteria, which belong to this group, are present in large numbers in the feces and intestinal tracts of humans and other warm-blooded animals, and can enter water bodies from human and animal waste. If a large number of fecal coliform bacteria (over 200 colonies/100 millilitres (ml) of water sample) are found in water, it is possible that pathogenic organisms are also present in the water.
Fecal coliform by themselves are usually not pathogenic; they are indicator organisms, which means they may indicate the presence of other pathogenic bacteria. Pathogens are typically present in such small amounts it is impractical monitor them directly. Swimming in waters with high levels of fecal coliform bacteria increases the chance of developing illness (fever, nausea or stomach cramps) from pathogens entering the body through the mouth, nose, ears, or cuts in the skin.
Diseases and illnesses that can be contracted in water with high fecal coliform counts include typhoid fever, hepatitis, gastroenteritis, dysentery and ear infections. Fecal coliform, like other bacteria, can usually be killed by boiling water or by treating it with chlorine. Washing thoroughly with soap after contact with contaminated water can also help prevent infections.
Fecal coliform, like other bacteria, can usually be killed by boiling water or by treating it with chlorine. Washing thoroughly with soap after contact with contaminated water can also help prevent infections.
Measurement of Fecal Coliform:
Bacteria are single-celled organisms that can only be seen with the aid of a very powerful microscope. However, coliform bacteria form colonies as they multiply, which may grow large enough to be seen. By growing and counting colonies of coliform bacteria from a sample of water, it is possible to determine approximately how many bacteria were originally present. There are several ways coliform bacteria are grown and measured. Methods commonly used include the most probable number (MPN) method and the membrane filter (MF) method. In the MPN method, a “presumptive test” is performed first.
A series of fermentation tubes that contain lauryl tryptose broth are inoculated with the water sample and incubated for 24 hours at 35 °C. Fermentation tubes are arranged in 3 or more rows, with 5 or 10 tubes per row, with varying dilutions of the samples in the tubes. The fermentation tube contains an inverted tube to trap gases that are produced by the coliform bacteria. After 24 hours, the fermentation tube is examined for gas production. If there is no gas production, the samples are incubated for another 24 hours and reexamined.
If gas production is observed by the end of 48 hours, the presumptive test is positive; coliform bacteria are present in the sample. A “confirmed test” is then performed to determine if fecal coliform bacteria are present. For the confirmed test, some of the content of the fermentation tube is transferred with a sterile loop to a fermentation tube containing another broth.
The sample is incubated in a water bath at 44.5 °C for 24 hours. Gas production in the fermentation tube after 24 hours is considered a positive reaction, indicating fecal coliform. Based on which dilutions showed positive for coliform and/or fecal coliform, a table of most probable numbers is used to estimate the coliform content of the sample. The results are reported as most probable number (MPN) of coliform per 100 ml.
The MF method is more rapid than the MPN method, but the results are not as reliable for samples that contain many non-coliform bacteria, high turbidity, and/or toxic substances such as metals or phenols. The water sample is filtered through a sterile membrane filter. The filter is transferred to a sterile petri dish and placed on a nutrient pad saturated with broth.
The plates are inverted, placed in watertight plastic bags, and incubated in a water bath at 44.5 degrees C for 24 hours. Colonies produced by fecal coliform bacteria are blue, and are counted using a microscope or magnifying lens. The fecal coliform density is recorded as the number of organisms per 100 ml.
Sometimes the unit of colony producing units per 100 millilitres of water (CPU/100 ml) is used; this is equal to the number of organisms per 100 ml.
Factors Affecting Fecal Coliform:
(i) Wastewater and Septic System Effluent:
Fecal coliform is present in human waste, so the bacteria goes down the drains in our houses and businesses, and can enter streams from illegal or leaky sanitary sewer connections, poorly functioning septic systems, and poorly functioning Wastewater Treatment Plant (WWTP) effluent.
(ii) Animal Waste:
A significant amount of fecal coliform is released in the wastes produced by animals. This can be a serious problem in waters near cattle feedlots, hog farms, dairies, and barnyards that have poor animal keeping practices and waste is not properly contained. In urban areas, fecal coliform can be contributed to surface water by dog, cat, raccoon, and human waste when it is carried into storm drains, creeks, and lakes during storms.
(iii) Sediment Load:
High amounts of sediment are often related to high concentrations of pathogenic bacteria. The bacteria can attach to sediment particles, escaping invertebrate predators. Fast-running water can carry more sediment, so higher levels of bacteria can occur during high run-off events. Bacteria are much more abundant on soils than in water.
(iv) Temperature:
Bacteria grow faster at higher temperatures. The growth rate slows drastically at very low temperatures.
(v) Nutrients:
High levels of nutrients can increase the growth rate of bacteria.
Factor # 11. Turbidity:
Turbidity is a measure of the cloudiness of water- the cloudier the water, the greater the turbidity. Turbidity in water is caused by suspended matter such as clay, silt, and organic matter and by plankton and other microscopic organisms that interfere with the passage of light through the water. Turbidity is closely related to total suspended solids (TSS), but also includes plankton and other organisms.
Turbidity itself is not a major health concern, but high turbidity can interfere with disinfection and provide a medium for microbial growth. It also may indicate the presence of microbes.
Measurement of Turbidity:
Turbidity is a measure of how much of the light travelling through water is scattered by suspended particles. The scattering of light increases with increasing suspended solid and plankton content. Turbidity in slow moving, deep waters can be measured using a device called a Secchi disk. A Secchi disk is a black and white, 20 cm diameter disk. The disk is lowered into the water until it just disappears from sight. The depth at which the disk disappears is called the Secchi depth, and is recorded in metres.
A Secchi disk does not work in shallow, fast-moving streams. In these waters, a turbidimeter is used. A turbidimeter measures the scattering of light, and provides a relative measure of turbidity in Nephelometric Turbidity Units (NTUs). A less expensive method of measuring turbidity is to evaluate the fuzziness of a mark at the bottom of a clear tube when a water sample is poured in the tube. Units are reported in Jackson Turbidity Units (JTUs). This method can only be used in highly turbid waters.
Factors Affecting Turbidity:
Because one of the primary factors affecting turbidity is total suspended solids, the factors affecting TSS will also affect turbidity.
In addition, organic matter contributes to turbidity:
(i) High Flow Rates:
The flow rate of a water body is a primary factor influencing turbidity concentrations. Fast running water can carry more particles and larger-sized sediment. Heavy rains can pick up sand, silt, clay, and organic particles from the land and carry it to surface water. A change in flow rate also can affect turbidity; if the speed or direction of the water current increases, particulate matter from bottom sediments may be re-suspended (WATERSHEDSS).
(ii) Soil Erosion:
Soil erosion is caused by disturbance of a land surface. Soil erosion can be caused by Building and Road Construction, Forest Fires, Logging, and Mining. The eroded soil particles can be carried by storm-water to surface water. This will increase the turbidity of the water body.
(iii) Urban Run-Off:
During storm events, soil particles and debris from streets and industrial, commercial, and residential areas can be washed into streams. Because of the large amount of pavement in urban areas, natural settling areas have been removed, and sediment is carried through storm drains to creeks and rivers.
(iv) Wastewater and Septic System Effluent:
The effluent from Wastewater Treatment Plants (WWTPs) can add suspended solids and organic material to a stream. The wastewater from our houses contains food residue, human waste, and other solid material that we put down our drains. Most of the solids and organic material are removed from the water at the WW TP before being discharged to the stream, but treatment can’t eliminate everything.
(v) Decaying Plants and Animals:
As plants and animals present in a water body die and decay, suspended organic particles are released and can contribute to turbidity.
(vi) Bottom-Feeding Fish:
Bottom-feeding fish (such as carp) can stir up sediments as they remove vegetation. These sediments can contribute to turbidity.
(vii) Algal Blooms:
Algal blooms can contribute to turbidity. Algal production is enhanced when nutrients are released from bottom sediments during seasonal turnovers and changes in water current. (WATERSHEDSS).
(viii) Flooding:
As flood waters recede, they will bring along inorganic and organic particles from the land surface, and contribute this to the stream.
Factor # 12. Total Suspended Solids (TSS):
The term “total solids” refers to matter suspended or dissolved in water or wastewater, and is related to both specific conductance and turbidity. Total solids is the term used for material left in a container after evaporation and drying of a water sample. Total Solids includes both total suspended solids, the portion of total solids retained by a filter and total dissolved solids, the portion that passes through a filter.
Total solids can be measured by evaporating a water sample in a weighed dish, and then drying the residue in an oven at 103 to 105 °C. The increase in weight of the dish represents the total solids. Instead of total solids, laboratories often measure total suspended solids and/or total dissolved solids. Total Suspended Solids (TSS) are solids in water that can be trapped by a filter. TSS can include a wide variety of material, such as silt, decaying plant and animal matter, industrial wastes, and sewage. High concentrations of suspended solids can cause many problems for stream health and aquatic life.
High TSS can block light from reaching submerged vegetation. As the amount of light passing through the water is reduced, photosynthesis slows down. Reduced rates of photosynthesis causes less dissolved oxygen to be released into the water by plants. If light is completely blocked from bottom dwelling plants, the plants will stop producing oxygen and will die.
As the plants are decomposed, bacteria will use up even more oxygen from the water. Low dissolved oxygen can lead to fish kills. High TSS can also cause an increase in surface water temperature, because the suspended particles absorb heat from sunlight. This can cause dissolved oxygen levels to fall even further (because warmer waters can hold less DO), and can harm aquatic life in many other ways, as discussed in the temperature section.
The decrease in water clarity caused by TSS can affect the ability of fish to see and catch food. Suspended sediment can also clog fish gills, reduce growth rates, decrease resistance to disease, and prevent egg and larval development. When suspended solids settle to the bottom of a water body, they can smother the eggs of fish and aquatic insects, as well as suffocate newly hatched insect larvae.
Settling sediments can fill in spaces between rocks which could have been used by aquatic organisms for homes. High TSS in a water body can often mean higher concentrations of bacteria, nutrients, pesticides, and metals in the water. These pollutants may attach to sediment particles on the land and be carried into water bodies with storm water. In the water, the pollutants may be released from the sediment or travel farther downstream.
High TSS can cause problems for industrial use, because the solids may clog or scour pipes and machinery.
Measurement of Total Suspended Solids:
To measure TSS, the water sample is filtered through a pre-weighed filter. The residue retained on the filter is dried in an oven at 103 to 105 °C until the weight of the filter no longer changes. The increase in weight of the filter represents the total suspended solids. TSS can also be measured by analyzing for total solids and subtracting total dissolved solids.
Factors Affecting Total Suspended Solids:
(i) High Flow Rates:
The flow rate of the water body is a primary factor in TSS concentrations. Fast running water can carry more particles and larger-sized sediment. Heavy rains can pick up sand, silt, clay, and organic particles (such as leaves, soil, tire particles) from the land and carry it to surface water. A change in flow rate can also affect TSS; if the speed or direction of the water current increases, particulate matter from bottom sediments may be re-suspended.
(ii) Soil Erosion:
Soil erosion is caused by disturbance of a land surface. Soil erosion can be caused by Building and Road Construction, Forest Fires, Logging, and Mining. The eroded soil particles can be carried by storm water to surface water. This will increase the TSS of the water body.
(iii) Urban Run-Off:
During storm events, soil particles and debris from streets and industrial, commercial, and residential areas can be washed into streams. Because of the large amount of pavement in urban areas, infiltration is decreased, velocity increases, and natural settling areas have been removed. Sediment is carried through storm drains directly to creeks and rivers.
(iv) Wastewater and Septic System Effluent:
The effluent from Wastewater Treatment Plants (WWTPs) can add suspended solids to a stream. The wastewater from our houses contains food residue, human waste, and other solid material that we put down our drains. Most of the solids are removed from the water at the WWTP before being discharged to the stream, but treatment can’t eliminate everything.
(v) Decaying Plants and Animals:
As plants and animals decay, suspended organic particles are released and can contribute to the TSS concentration.
(vi) Bottom-Feeding Fish:
Bottom-feeding fish (such as carp) can stir up sediments as they remove vegetation. These sediments can contribute to TSS.
Factor # 13. Total Dissolved Solids (TDS):
Total Dissolved Solids (TDS) are solids in water that can pass through a filter (usually with a pore size of 0. 45 micrometers). TDS is a measure of the amount of material dissolved in water. This material can include carbonate, bicarbonate, chloride, sulfate, phosphate, nitrate, calcium, magnesium, sodium, organic ions, and other ions.
A certain level of these ions in water is necessary for aquatic life. Changes in TDS concentrations can be harmful because the density of the water determines the flow of water into and out of an organism’s cells. However, if TDS concentrations are too high or too low, the growth of much aquatic life can be limited, and death may occur.
Similar to TSS, high concentrations of TDS may also reduce water clarity, contribute to a decrease in photosynthesis, combine with toxic compounds and heavy metals, and lead to an increase in water temperature.
TDS is used to estimate the quality of drinking water, because it represents the amount of ions in the water. Water with high TDS often has a bad taste and/or high water hardness, and could result in a laxative effect.
Measurement of Total Dissolved Solids:
To measure TDS, the water sample is filtered, and then the filtrate (the water that passes through the filter) is evaporated in a pre-weighed dish and dried in an oven at 180° C, until the weight of the dish no longer changes. The increase in weight of the dish represents the total dissolved solids, and is reported in milligrams per litre (mg/1).
The TDS concentration of a water sample can be estimated from specific conductance if a linear correlation between the two parameters is first established. Depending on the chemistry of the water, TDS (in mg/1) can be estimated by multiplying specific conductance (in micromhos/cm) by a factor between 0.55 and 0.75.
TDS can also be determined by measuring individual ions and adding them up.
Factors Affecting Total Dissolved Solids:
(i) Geology and Soil in the Watershed:
Some rock and soil release ions very easily when water flows over them; for example, if acidic water flows over rocks containing calcite (CaCO3), such as calcareous shales, calcium (Ca2+) and carbonate (CO32-) ions will dissolve into the water. Therefore, TDS will increase. However, some rocks, such as quartz-rich granite, are very resistant to dissolution, and don’t dissolve easily when water flows over them. TDS of waters draining areas where the geology only consists of granite or other resistant rocks will be low (unless other factors are involved).
(ii) Urban Run-Off:
During storm events, pollutants such as salts from streets, fertilizers from lawns, and other material can be washed into streams and rivers. Because of the large amount of pavement in urban areas, natural settling areas have been removed, and dissolved solids are carried through storm drains to creeks and rivers.
(iii) Fertilizer Run-Off:
Fertilizer can dissolve in storm-water and be carried to surface water during storms, and contribute to TDS.
(iv) Wastewater and Septic System Effluent:
The effluent from Wastewater Treatment Plants (WWTPs) adds dissolved solids to a stream. The wastewater from our houses contains both suspended and dissolved solids that we put down our drain. Most of the suspended solids are removed from the water at the WVVTP before being discharged to the stream, but WWTPs only remove some of the TDS. Important components of the TDS load from WWTPs include phosphorus, nitrogen, and organic matter.
(v) Soil Erosion:
Soil erosion is caused by disturbance of a land surface. Soil erosion can be caused by Building and Road.
(vi) Construction, Forest Fires, Logging, and Mining:
The eroded soil particles may contain soluble components that can dissolve and be carried by storm water to surface water. This will increase the TDS of the water body.
(vii) Decaying Plants and Animals:
As plants and animals decay, dissolved organic particles are released and can contribute to the TDS concentration.