Here is a compilation of essays on ‘Acid Rain’ for class 6, 7, 8, 9, 10, 11 and 12. Find paragraphs, long and short essays on ‘Acid Rain’ especially written for school and college students.
Essay on Acid Rain
Essay Contents:
- Essay on the Introduction to Acid Rain
- Essay on the Chemistry of Acid Rain
- Essay on the Wet Acid Rain
- Essay on the Areas of Acid Rain
- Essay on the Adverse Effects of Acid Rain
- Essay on the Solutions to Acid Rain
- Essay on the Measures to Control Acid Rain
Essay # 1. Introduction to Acid Rain:
Acid rain was first reported by Scottish scientist Robert Angus Smith in 1852. However the term ‘acid rain’ came into existence in 1972. Acid rain is the manifestation of serious air pollution. The gases emitted from various sources mix with the rain to form an acidic precipitation, called acid rain. This acidic precipitation is capable of threatening the balance of entire ecological cycle. Acid rain affects all the living beings directly or indirectly. The future implications of acid rains can be severe, if proper measures are not taken on time.
Any form of precipitation which is acidic in nature is called acid rain. Acid rain is the result of excessive emissions of sulfur and nitrogen caused by human activity, which reacts with other compounds to form acids. Acid rain has detrimental effects on animals, plants and infrastructure.
In its purest state, rain water is like distilled water. It does not have carbon dioxide dissolved in it. It is neutral, with a pH level of 7. pH is the concentration of hydrogen ions in an aqueous solution. If the pH level is above 7, it is said to be basic, and if it is below 7, it is said to be acidic in nature.
As rain water falls through the atmosphere, particles suspended in the air are dissolved in it. These substances are generally dust, pollen grains and carbon dioxide (CO2). Emissions of volcanoes and lightning tend to decrease the pH level of acid rain, making it even more acidic. CO2 combines with water to form carbonic acid (H2CO3).
H2O(l) + CO2(g) = H2CO3(aq)
Carbonic acid ionizes in water to form low concentrations of carbonate and hydronium ions.
2H2O(l) + H2CO3(aq) = CO32-(aq) + 2H3O+(aq)
Carbonic acid is a weak acid. It brings down the pH of the rain water to 6.0-5.2. With pH levels ranging between 6.0-5.2, rain water is acidic, but still not dangerous. This is a reversible reaction.
The problem occurs when rain water combines with gaseous oxides of sulphur, nitrogen, and phosphoric and hydrochloric acid mists. The latter two and sulphur are released into the atmosphere from automobile exhausts, industries and electric power plants. Nitrogen forms a major part of atmospheric composition. These chemicals bring down the acid rain pH level to 5.6-3.5. Sometimes, the pH level can even become as low as 2. This phenomenon of acidic rain water precipitation is called acid rain. Rain, snow, sleet, freezing rain, hail, fog and dew are other forms of precipitation.
Essay # 2. Chemistry of Acid Rain:
Sulphuric acid and nitric acid are the main acids present in acid rain. Sulfuric acid is formed as follows:
(i) Sulphur released into the atmosphere combines with atmospheric oxygen to form sulfur dioxide (SO2)
(ii) Sulphur dioxide reacts with atmospheric water to form sulphurous acid – SO2(g) + H2O(l) = H2SO3(aq)
(iii) Sulphurous acid is also present in acid rain.
(iv) Sulphur dioxide gradually oxidizes to form sulphur trioxide (SO3) – 2SO2(g) = O2(g) = 2SO3(g)
(v) Sulphur trioxide reacts with water to form Sulphuric acid (H2SO4) – SO3(g) + H2O(l) = H2SO4(aq) Nitrogen dioxide(NO2) is formed as follows:
(vi) Nitrogen combines with atmospheric oxygen to form nitrogen dioxide (NO2). Nitrogen dioxide reacts with water to form nitrous acid (HNO2) and nitric acid (HNO3) – 2NO2(l) + H2O(l) = HNO2(aq) + HNO3(aq)
Acid rain is a mild combination of mainly sulfuric and nitric acid. Sulphurous acid and nitrous acid are less stable and are present only in very low amounts. Following are the various adverse effects of acid rain on living organisms and infrastructure.
Essay # 3. Wet Acid Rain:
Every source of energy (coal, fuel, wood or petroleum products) has sulphur and nitrogen. These two elements, when burnt in atmospheric oxygen, are converted into their respective oxides (SO2 and NO2), which are highly soluble in water.
Under the humid conditions of air, N2 O5 invariably reacts with water vapours and form droplets of HNO3–
N2O5 + H2O → 2HNO3
Some HNO2 is also formed.
N2O3 + H2O → 2HNO2
HNO3 and HNO2 then return to the earth’s surface. However, HNO3 can be removed as a particulate or as particulate nitrate after reaction with bases such as NH3.
SO2 in humid atmosphere forms droplets of H2SO4.
Presence of hydrocarbon and NOx steps up the oxidation rate of reaction. In water droplets, ions such as Mn(II), Fe(II), Ni(II) and Cu(II) catalyze the oxidation reaction.
HNO3 and H2SO4 thus formed combine with HCl (emitted from natural and manmade sources) to generate precipitation, which is commonly referred to as acid rain. Normally unpolluted rain is weakly acidic and has a pH of 5.6
Oxidation of Sulphur Dioxide:
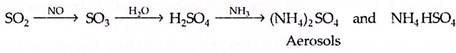
The foremost chemical reaction of SO2 in air is its oxidation to SO3 which reacts with the moisture to give H2SO4. The H2SO4 or its sulphates occur as aerosols—
Oxidation step proceed in three ways:
1. Catalytic oxidation.
2. Photochemical oxidation.
3. Oxidation by radicals.
Essay # 4. Areas of Acid Rain:
In highly populated cities, internal combustion engines, used by masses, emit pollutants like CO2, CO, SO2, NO, NO2, etc. on road around them in atmosphere, which are absorbed by moisture present in air forming various acids, e.g., H2SO4, HCl, and HNO3 etc. Thus more acid rains are likely to occur in such areas where there is tendency of formation of acids due to absorption of various gases by the moisture present in the environment-
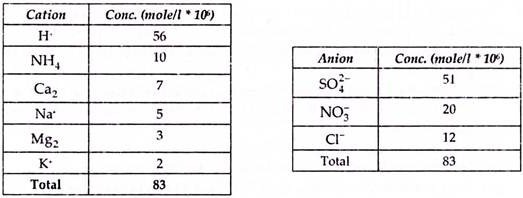
A typical sample of acid rain contain following cations and anions.
Essay # 5. Adverse Effects of Acid Rain:
Acid rain exerts both direct and indirect effect on the organisms and materials. The direct effects of acid rain are determined by concentration of pollutants in the air. They are mainly of local nature with a geographical extent of few kms. They decline rapidly with distance from the emission source. They affect organisms and materials and cause more harm at a distance up to hundreds and sometimes thousands of kms.
The dry deposition has several direct effects on the environment. It attacks building materials, mainly, limestone, sandstone, marble, steel, nickel and other metals causing a loss of millions of rupees. When deposited in gaseous form it causes direct damage to plants and trees, the visible injury being gradual yellowing or depigmentation of leaf tissues, i.e., chlorosis.
The wet deposition has direct as well as indirect effects. It increases the acidity of lakes and rivers which is made worse by inflow of acids and metals from nearby soils. Wet deposition also affects aquatic as well as terrestrial ecosystems. Wet deposition can transport metals such as Al, Cd, Hg and Cr into soil water, ground water, lakes and streams, depleting the stocks of nutrients in the soil, thereby causing harm to various ecosystems. Acidity kills fish, algae, bacteria and aquatic system gets collapsed into the sterility leaving a crystal clear but ultimately a dead lock.
The increased acidity of rainwater causes damage of fresh water life. In the form of mist it causes the damage of plant leaves, changes the rate of metabolism of organisms. These also produce irritation to eyes and mucous membrane. This acid rain accelerates the rate of corrosion. It dissolves salts in the soil (e.g., CaCO3) and metals like aluminium, which passes in rivers where they cause toxic effects to aquatic life forms.
(a) Effects of Acid Rain on Aquatic Biota:
1. A significant reduction in fish population accompanied by decrease in the variety of species in food chain has been observed.
2. Many bacteria and blue green algae are killed due to the acidification, disrupting the whole ecological balance. Acid rain killed fishes in lakes and destroyed trees in a wide swathe across Europe.
(b) Effect of Acid Rain on Terrestrial Ecosystem:
1. In West Germany, about 13% of the forests died and nearly 20 million acres of forests are affected by acid rains. Forests in Switzerland, Netherlands and Czechoslovakia have been damaged by such rains, Actually nutrients like calcium, potassium, iron and magnesium have been away from soil by acids. These nutrients are most essential for the plant growth.
2. The effect of acid precipitation on terrestrial vegetation indicates reduced rate of photosynthesis and growth and increased sensitivity to drought and disease.
(i) Acid deposition weakens the trees like pine, which can be easily attacked by pathogens and drought.
(ii) Acidification of soil changes biology and chemistry. Plants can easily absorb Cd from the acidified soil. High levels of Cd in plants are injurious for animals and human beings.
(iii) Root systems are damaged by the uptake of aluminium released from the soil. Nitrates may be released from the soil by acid run-off waters.
(iv) Trees pull heavy metal ions, like Cd, Cr and Al into their systems.
These ions are imunobile in many soils, but acidic water mobilizes them and cause tree damage.
(v) Acid rain has severely retarded the growth of crop such as pea, beans, potato, broccoli and carrots etc.
(c) Effects of Acid Rain on Lake Ecosystem:
1. The activity of the bacteria and other microscopic animals is reduced in acidic water. So the dead materials and other accumulated substances lying on the bottom of lakes are not rapidly decomposed. Thus essential nutrients as nitrogen and phosphorus stay locked up in plant and animal remains. Biomass production is reduced and fish population declines.
2. Acid rains cause a number of complications in ponds, rivers and lakes where it accumulate as acid snow. In summer rapid snowmelt gives a jolt of acid water to lakes. This acid fell is most damaging to young fish, algae, and insects and to the food chain.
3. Black flies, mosquitoes, deer flies and other aquatic worms occur abundantly where fishes are eliminated. So they appear to thrive in acid conditions. Dragon fly larvae and water boatmen also flourish in acidified lakes.
4. Aquatic plants such as broad-leafed pondweeds do not grow in acidic water. This could affect the feeding and breeding habits of aquatic species.
(d) Effects of Acid Rain on Human Beings:
1. Acid rain has been found to be very dangerous to the living organisms as it can destroy life. Acidification can play havoc with human nervous system, respiratory system and digestive system by making the person an easy prey to neurological diseases. This happens because these acids produce highly toxic compounds which contaminate the potable water and enter man’s body.
2. Acidic rains containing air pollutants contribute to variety of safety hazards, associated with reduced visibility due to smog etc. These contaminants can be nuisance in several aspects and cause adverse health effects.
(e) Effects of Acid Rain on Buildings:
1. Stone leprosy — Acid rain causes extensive damage to buildings and structural materials of marble, limestone, slate and mortar etc. Limestone is attacked rapidly.
CaCO3 + H2SO4 → CaSO4 + H2O + CO2
The attack on marble is termed as stone leprosy.
2. The Taj Mahal in Agra is also suffering at present due to SO2 and H2SO4 acid fumes or air pollutants released from Mathura refinery.
3. Due to acidity, levels of heavy metals as Al, Mn, Pb, Cd, Cr and Cu in water increases beyond the safe limit which indirectly affects the buildings.
4. H2S tarnishes silver and blackens leaded house paints. Ozone produces cracks in rubber. Trace of radioactive elements present in radioactive rain severely damages the buildings.
Essay # 6. Solutions to Acid Rain:
Acid rain is potent enough to destroy life on Earth. It damages anything it comes in contact with. It ruins forests, water bodies, soil, infrastructure, and the health of living beings. It’s high time we take all possible measures to control it. There is a solution to every problem, and acid rain is no exception.
Take a look at the various measures we can take:
(i) Human beings should reduce the use of fossil fuels. This would lessen the emission of nitrogen and sulfur in the environment.
(ii) Public transportation, car pools and walking can reduce nitrogen, sulfur and lead emissions into the atmosphere.
(iii) Sulphur and nitrogen are mostly released into the atmosphere from the burning of fossil fuels (e.g., Coal). Switching over to alternative forms of energy such as geothermal, water, wind, and solar power would help to a great extent.
Ail of the above measures are simple steps that can easily be adopted on an individual level. Acid rain has become an international issue because of its serious and definite danger to life on earth. Many international treaties have been signed i.e., the Sulfur Emissions Reduction Protocol and the Convention on Long-Range Trans-boundary Air Pollution. Installing Flue Gas Desulfurization e.g., wet scrubber in coal-burning power plants to remove sulfur-containing gases – is one of the steps taken by the USA, and is followed by a number of developed and developing countries.
Essay # 7. Measures to Control Acid Rain:
Though the havoc played by acid rain cannot be reversed, certain actions can be taken to avoid further damage. General awareness among common people and pressure from various environmental groups have forced the Governments to take measures against the acid rain. Lakes and streams can regain their PH balance by the process of liming. Liming means dissolving large quantities of alkaline substances like quicklime into the water bodies. In addition, measures like reducing power consumption, cutting down on fuel and afforestation can also bring down the pollution level to a great extent.
There is an urgent need for proper and regular monitoring to provide timely warnings about acidification of our environment. Short-terms control of acid deposition problem can be achieved by using lime.
With air pollution a serious cause of concern in European countries, the European Union (EU) Environment Ministers have agreed in early March 1996 to reduce diesel fuel emissions all over Europe, because diesel emissions contain nitrogen oxides, which are known to cause acid rain and also diesel particles, some of which are carcinogenic in nature. Most of the machinery covered under the proposals account for about 30% of total nitrogen oxide emissions from diesel vehicles. The proposal was to reduce nitrogen oxide emission by 42% and diesel particles by 67% by the end of the year 2003.
Various chemical industries and automobiles release acidic oxides (like SO2, NO2, HCl etc.) into the atmosphere. These oxides and HCl dissolve in moisture present in atmosphere etc. form corresponding acids, which then fall snowing on earth as acid rain.
SO2+ H2O → H2SO3 Sulphurous acid
2SO2 + O2 + 2H2O → 2H2SO4 Sulphuric acid
4NO2 + 2H2O + O2 → 4HNO3 Nitric acid
HCl + H2O → HCl(aq.) Hydrochloric acid
Automobiles (consisting of internal combustion engines) exit pollutants like CO2, CO, hydrocarbons etc.; while large number of industries exit pollutants like CO2, CO, SO, SO2, NO, NO2 etc. in the atmospheric air, which are absorbed by moisture, present in air, forming corresponding acids.
Some mechanical or chemical methods should be adopted by the industries and automobiles to prevent the formation of harmful pollutants and gases, which may cause acid rains. Combustion engines should be so manufactured that they do not release harmful pollutants in the atmosphere, which may be absorbed by the moisture present in the environment causing acid rain.