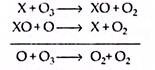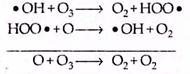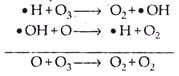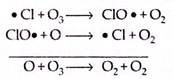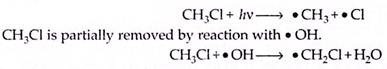Here is a compilation of essays on ‘Ozone Layer Depletion’ for class 6, 7, 8, 9, 10, 11 and 12. Find paragraphs, long and short essays on ‘Ozone Layer Depletion’ especially written for school and college students.
Essay on Ozone Layer Depletion
Essay Contents:
- Essay on the Introduction to Ozone Layer Depletion
- Essay on the Causes of Ozone Layer Depletion
- Essay on the Mechanism of Ozone Layer Depletion
- Essay on the Antarctic and Arctic Ozone Hole Formation
- Essay on the Effects of Ozone Layer Depletion
- Essay on the Solution to Ozone Layer Depletion
Essay # 1. Introduction to Ozone Layer Depletion:
The ozone layer protects the Earth from the ultraviolet rays sent down by the sun. If the ozone layer is depleted by human action, the effects on the planet could be catastrophic.
Ozone is present in the stratosphere. The stratosphere reaches 30 miles above the Earth, and at the very top it contains ozone. The suns rays are absorbed by the ozone in the stratosphere and thus do not reach the Earth.
Ozone is a bluish gas that is formed by three atoms of oxygen. The form of oxygen that humans breathe in consists of two oxygen atoms, O2. When found on the surface of the planet, ozone is considered a dangerous pollutant and is one substance responsible for producing the greenhouse effect.
The highest regions of the stratosphere contain about 90% of all ozone.
In recent years, the ozone layer has been the subject of much discussion. And rightly so, because the ozone layer protects both plant and animal life on the planet.
The fact that the ozone layer was being depleted was discovered in the mid-1980s. The main cause of this is the release of CFCs, chlorofluorocarbons.
Antarctica was an early victim of ozone destruction. A massive hole in the ozone layer right above Antarctica now threatens not only that continent, but many others that could be the victims of Antarctica’s melting icecaps. In the future, the ozone problem will have to be solved so that the protective layer can be conserved.
Essay # 2. Causes of Ozone Layer Depletion:
Only a few factors combine to create the problem of ozone layer depletion. The production and emission of CFCs, chlorofluorocarbons, is by far the leading cause.
Many countries have called for the end of CFC production because only a few produce the chemical. However, those industries that do use CFCs do not want to discontinue usage of this highly valuable industrial chemical.
CFCs are used in industry in a variety of ways and have been amazingly useful in many products. Discovered in the 1930s by American chemist Thomas Midgley, CFCs came to be used in refrigerators, home insulation, plastic foam, and throwaway food containers.
Only later did people realize the disaster CFCs caused in the stratosphere. There, the chlorine atom is removed from the CFC and attracts one of the three oxygen atoms in the ozone molecule. The process continues, and a single chlorine atom can destroy over 100,000 molecules of ozone.
In 1974, Sherwood Rowland and Mario Molina followed the path of CFCs. Their research proved that CFCs were entering the atmosphere, and they concluded that 99% of all CFC molecules would end up in the stratosphere.
Only in 1984, when the ozone layer hole was discovered over Antarctica, was the proof truly conclusive. At that point, it was hard to question the destructive capabilities of CFCs.
Even if CFCs were banned, problems would remain. There would still be no way to remove the CFCs that are now present in the environment. Clearly though, something must be done to limit this international problem in the future.
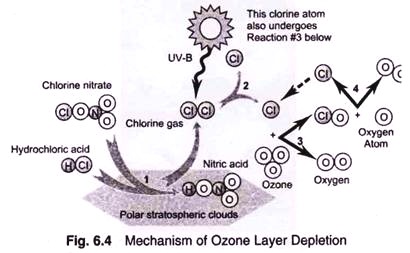
Essay # 3. Mechanism of Ozone Layer Depletion:
It includes, 1. The natural process. 2. The anthropogenic process.
1. The Natural Process:
Atmospheric oxygen absorbs ultraviolet radiation shorter than 240 nm and photo-dissociates into two oxygen atoms. These unite with other O2 molecules to form ozone. During the process surplus energy of nascent O3 is often transferred to the nearby molecules as kinetic energy which slightly raises the surrounding atmospheric temperature. Ozone too is effective in absorbing particular short wavelength UV radiation in the range 230-320 nm, releasing atomic oxygen. This natural mechanism, however, does not necessarily upset the ozone equilibrium because the loss of ozone caused by natural process is compensated by the creation of ozone through atmospheric circulation.
Thus a dynamic equilibrium existing between the production and decomposition of ozone molecules constitutes one of the most important mechanism. The heat generated during the reaction causes a rise in temperature of the stratosphere. Secondly, the photochemical process absorbs most of the harmful solar ultraviolet radiations of wavelength from 2000 Å to 3500 Å.
Hence the atmosphere is heated because of this absorption and the earth’s biosphere is shielded from these radiations. Without it, life on the earth would be completely destroyed. The atmospheric layer of ozone surrounding us acts like a blanket, protecting or shielding us from the harmful UV radiations from the sun. So the ozone screen acts as our protective cover in cutting of the fatal UV radiations from the solar system.
Ozone acts as a powerful oxidant because of its ability to remove electrons from other molecules. It occurs as a natural component of air. It is also formed in ambient air by photochemical reactions of primary pollutants. Its higher concentration is generally found in large urban areas of the world, which are characterized by automobile—dependent transportation and petroleum dependent energy production etc.
Surface ozone is produced as a result of chemical reactions in the atmosphere driven by the action of UV radiation in the sunlight, involving both nitrogen oxides (NOx) and volatile organic matter (VOM).
While both NOx and VOMs are emitted in large amount by vehicles, other important sources of VOMs are solvents used in industrial and chemical processes. According to British scientists, this surface ozone is responsible for the substantial reduction in the yields of major crops like wheat and rice. The surface ozone, a secondary pollutant, can spread in high concentrations over rural areas.
There are the other sources of ozone in the environment, such as gamma radiation used in food preservation plants, commercial UV lamps used for sterilization, high voltage electric equipments, dermatological phototherapy equipments and even in photocopying machines. Ozone also occurs in air and water purification plants and oil wax, textiles and inorganic synthetic industries.
2. The Anthropogenic Process:
We know that there are natural chemical and photochemical processes which lead to the continuous formation and destruction of ozone in the stratosphere. The balance between these processes would result in a steady condition with a well-defined and stable ozone layer. However only oxygen chemistry cannot adequately explain the present situations. Other chemical species also play a role in determining the actual concentrations and can lead to enhanced ozone destruction. Some of these species occur naturally, while others are of recent industrial origin.
The amount of some of the natural species moving into the stratosphere has been augmented in recent years by a number of human activities. Many of the processes, which are responsible for the depletion of ozone layer, share a general mechanism of the type,
Net reaction
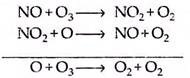
Here X is the reacting species. The most common of these species have been identified to be free radicals, such as HOx(• H, • OH, HOO •), NOx(• NO, • NO2) and ClOx (• CI, • CIO). Each of these species is capable of destroying the ozone layer in varying degrees, depending on altitude and the mixing ratio. For example, near the stratosphere the HOx radicals are responsible for about 70% of the total mechanism of ozone destruction, including the oxygen only process.
Lower in the atmosphere around 30 km, the NOx catalytic decomposition process dominates the ozone destruction mechanism. The NOx cycle in the region just above the troposphere has been found to be responsible for about 70% of the ozone destruction, but recent studies show that this figure may be even more.
(a) The hydroxyl radical (HOx) cycle:
The HOx radicals are generated by either of the following two photochemical reactions:
where O is excited state oxygen.
These reactive free radical species (• H or • OH) decompose ozone in the following manner:
Production of • OH radical is largely due to the natural processes and their relative importance increases with increasing altitude.
The hydrogen radical decomposes the ozone in the following way:
(b) The nitric oxide (NOx) cycle:
The nitric oxide cycle contributes in a significant way in the natural destruction of stratospheric ozone in the lower region of the stratosphere:
In the upper atmosphere, NO is produced by reactions involving atomic and ionic species of nitrogen and oxygen. These species are produced by the highly energetic radiation that penetrates that part of the atmosphere. Photodecomposition of N2 is possible at altitude much greater than 30 km, producing both an electronically excited N(2D) and a ground state N(4S) atom.
The ground state nitrogen subsequently reacts with O2, producing another natural source of NO:
At altitudes of less than 30 km, additional NO is also generated via a reaction:
N2O + O(1D) → 2NO
Where O(1D) is excited state oxygen. The principal source of nitrous oxide is the process of denitrification in soils having high concentration of nitrate ion:
The increase in atmospheric nitrous oxide (N2O) mixing rations is due (to some extent) to enhanced rates of denitrification resulting from greater use of nitrogenous fertilizers worldwide.
Although the mixing ratio of nitrous oxide in the troposphere is steadily increasing, the production of stratospheric nitric oxide through various processes can, in a large part, be attributed to natural causes. Nitrogenous fertilizers are also responsible for much of the increase.
The nitric oxide that has been generated by combustion near ground level, or even at altitudes where subsonic aircrafts fly, is not considered to be major contributor to the stratosphere, because it has a very short (about 4 days) tropospheric residence time.
(c) Chlorine radical (ClOx) cycle:
The CFCs and halons are entirely manmade with wide applications in air conditioning, refrigeration, aerosols, electric and metal clearing, foam blowing and modern firefighting. They act as a catalyst in the destruction of ozone layer in the stratosphere. The rate of destruction of ozone is further enhanced by the oxides of nitrogen (NOx) released from the exhausts of large fleet of supersonic aircrafts (SST). A wide range of chlorofluorocarbons (CFCs) are known, but widely used are CFCl3 and CF2Cl2, which are in use since 1930.
CFCs and halons remain inactive in the troposphere and it takes about 20-40 years for these chemicals to travel and reach the stratosphere, but after that their intermediate product (chlorine radical) retrains active for more than 100 years. The travelling time for CFCs and halons to reach to stratosphere may range from 20 to 40 years, that is, CFCs generated in the troposphere today may take 20 to 40 years to reach the stratosphere. Once these CFCs and halons reach to stratosphere, the chlorine and bromine atoms present in these chemicals are released as a result of interaction with UV radiations in the stratosphere.
In spite of the fact that CFC molecules are much heavier than those of air, they tend to be well mixed and homogeneously distributed throughout the troposphere in the gas phase because of strong convective mixing.
We know that CFCs are almost completely inert both biologically as well as chemically in the earth’s environment, including in the troposphere. Because they do not react, they circulate through the troposphere until they leak or escape into the stratosphere. At higher altitudes they are capable of undergoing UV photolytic decomposition, as a result of being exposed to the intense flux of energetic UV radiation—
CFCI3 + hv (λ< 290 nm) → • CFCl2 + • CI
The released chlorine radical is capable of taking part in the catalytic decomposition of ozone as follows:
A second and perhaps third chlorine radical could also be produced by further decomposition of • CFCl2 radical, generating additional • CI radical responsible for ozone depletion—
Chlorine atoms catalyse the destruction of ozone. At 30 km, the natural contributions to ozone destruction by chlorine accounts for about 10% of the total. A significant natural precursor of atomic chlorine is CH3Cl, which is produced biogenically from the oceans, from the burning of vegetation and from volcanic emissions. Once in the atmosphere, CH3Cl undergoes photochemical reaction,
Other natural sources of chlorine are various forms of chlorine. Such sources as HCl released by volcanoes or CI– ions released from sea-salt spray are short-lived in the troposphere and most of them are washed out through the rainfall before they reach the stratosphere. There are other catalytic cycles also involving chlorine. For example,
Bromine (from, say halons) can also replace chlorine in the cycle and together these reactions account for about 60% of the routes by which ozone is destroyed by halogens. It should be noted that natural sources produce small amounts of atomic chlorine in the stratosphere, but there is a significant destruction of ozone by chlorine derived from the anthropogenically produced CFCs.
Taking into consideration the ozone depleting CFCs and halons, scientists are trying to modify the relative amounts of fluorine, chlorine and hydrogen in new compounds. Thus a common approach is to incorporate hydrogen in the structure, as in hydrochlorofluorocarbons (HCFCs) or replace chlorine and produce hydrofluorocarbons(HFCs). Increasing the content of hydrogen reduces the inertness of the compound and in this way the lifetime of the compound in troposphere may be made short.
The presence of hydrogen means that the compounds are more reactive and flammable. But the higher reactivity is a disadvantage in many applications. If the proportion of fluorine is increased at the expense of chlorine, then a highly stable compound will be formed because the bond energy of C-F bond is larger than C-Cl bond. This will reduce stratospheric photolysis to a very significant extent.
Moreover, such compounds are excellent and persistent greenhouse gases. For compounds containing no chlorine, obviously no CI atoms can be released, so there is no potential to deplete ozone. Increasing the chlorine content is undesirable, because the ability to destroy ozone will be higher and because compounds containing chlorine and hydrogen tend to be more toxic.
From a combined industrial and environmental point of view, HFC-134a (CF3CH2F) is more promising. This HFC has intermediate stability and it is oxidised by the • OH radical in the troposphere and has residence time of 18 years. It is neither very flammable nor have chlorine. So it is not expected to have ozone depleting potential. HCFC-123 (CF3CHCl2) is another replacement for CFC. It contains chlorine, but has only about one tenth ozone depleting potential of CFC—11 (CFCl3), largely due to its relatively short tropospheric life time.
Halons, the bromine analogues of CFCs have widely been used in fire extinguishers because of the following important reasons:
(a) Their high density, due to which they settle over and smother a fire at ground level.
(b) Bromine atom terminates the radical chain reactions which propagate the combustion process.
Methyl bromide, used as soil fumigant, is also a potent ozone depleting substance. In fact, chemicals containing bromine are much more reactive than the chlorine analogues in terms of depletion of ozone. Because of this, ODP values for bromine compounds are very high. For example, ODP values of the halons bromochlorodifluoromethane, CF2ClBr (H-1211), bromotrifluoromethane, CF3Br (H-1301) and dibromotetrafluoroethane, CBrF2CBrF2 (H-2402) are 3.0, 10.0 and 6.0 respectively.
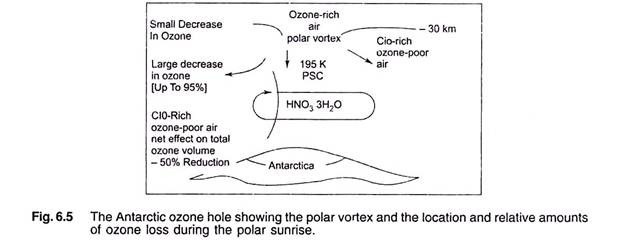
Essay # 4. Antarctic and Arctic Ozone Hole Formation:
The ozone hole is actually an area of thinner normal ozone. Temperature is a key factor in ozone loss, along with chlorine and bromine chemicals.
In 2001, the Antarctic ozone hole reached a maximum size of more than 26.5 million sq.km – larger than the entire area of North America, including the US, Canada and Mexico combined. In the year 2000, it briefly approached 11.30 million sq. km. In 2002, ozone hole over Antarctica was not only much smaller than what it was in 2000 and 2001, but it has split into two. It was measured at 15.6 million sq. km. In September 2002, a figure well below the more than 24 million sq. km spread seen consecutively during the last six years. The hole’s total column ozone was measured below 220 dobson units.
Ozone depletion is an annual phenomenon over the South Pole during August and September when coldest temperatures are prevalent. Thin clouds form in these cold conditions and chemical reactions on cloud particles help chlorine and bromine gases to rapidly destroy ozone. By early October, temperatures typically start to warm and the ozone layer starts to recover.
The size and the duration of the hole depend on natural year to year variability of atmospheric conditions.
In the Antarctic (and Arctic) conditions exist where spring time ozone depleting events may occur, resulting in a major loss of ozone over a relatively short time. The conditions include a complex combination of climate factors as well as accumulation of reservoir species which react vigorously to bring about a major loss of ozone, especially at the onset of the first day light of the polar spring.
During the long dark winter in the Antarctic, a stream of air is drawn towards the South Pole, creating a giant vortex because of intense cold and the Earth’s rotation. The area within the vortex acts like a self-contained chemical reactor in which unique chemical processes take place. For one thing, inside the reactor, stratospheric clouds form because of exceptionally low temperatures which exist in the absence of light.
The clouds are classified into two types:
(a) The polar stratospheric clouds (PSC) are formed at about 193 K and consist of about 1 µm diameter particles of HNO3 and H2O in the ratio of 1: 3.
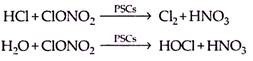
(b) The polar stratospheric clouds, which are formed when the temperature decreases to 187 K. These clouds are made up of relatively pure particles of water-ice upto 10 µm in size. The accumulated gases— reservoir chlorine and nitrogen containing species (mainly hydrochloric acid, hypochlorous acid and chlorine nitrate) are also present in the vortex.
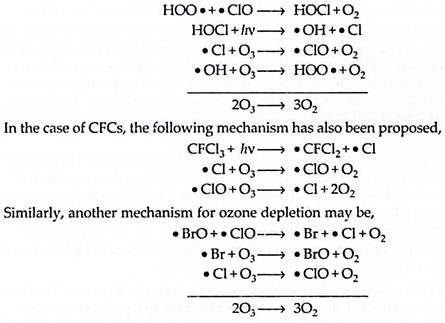
During the winter, these species take part in heterogeneous reaction on the surface of PSCs and consequently release chlorine molecules and HOCl:
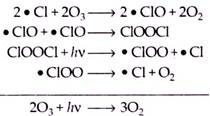
The relatively stable situation remains until after the onset of sunlight. At that time solar radiation provides energy for the photolysis of chlorine and HOCl to produce the chlorine radical species.
The chlorine radicals so formed are then available for ozone depletion by catalytic cycles or according to following reactions:
Depletion of ozone occurs rapidly so that even in some days, ozone levels decrease dramatically to half or even less of their winter value. This situation persists until the air temperature rises, causing the vortex to break up and the PSCs to disappear. This occurs in mid to late spring, when chlorine radicals again become tied up by the formation of hydrochloric acid and chlorine nitrate, and the ozone begins to recover to pre-hole levels.
In the Arctic atmosphere, as a result of the eruption of Mt Pinatubo in the Philippines during 1991, sulphate was found to be present at higher concentrations than usual. Stratospheric sulphate is usually in the form of an aerosol and acts as a catalyst for the removal of N2O5 by forming nitric acid
This reaction is a means by which NOx species are removed from the polar stratosphere, thus eliminating one of the species which is associated with holding cycles that tie up chlorine monoxide radicals (• CIO + • NO2 + M → ClONO2 + M). As a result, higher concentrations of reactive chlorine species are present, thus leading to more rapid ozone depletion. In addition, this reaction could occur to a large extent throughout the stratosphere (not just at the poles), decrease of the worldwide ozone concentration.
Essay # 5. Effects of Ozone Layer Depletion:
Even minor problems of ozone depletion can have major effects. Every time even a small amount of the ozone layer is lost, more ultraviolet light from the sun can reach the Earth.
Every time 1% of the ozone layer is depleted, 2% more UV-B is able to reach the surface of the planet. UV-B increase is one of the most harmful consequences of ozone depletion because it can cause skin cancer.
The increased cancer levels caused by exposure to this ultraviolet light could be enormous. The EPA estimates that 60 million Americans born by the year 2075 will get skin cancer because of ozone depletion. About one million of these people will die.
In addition to cancer, some research shows that a decreased ozone layer will increase rates of malaria and other infectious diseases. According to the EPA, 17 million more cases of cataracts can also be expected.
The environment will also be negatively affected by ozone depletion. The life cycles of plants will change, disrupting the food chain. Effects on animals will also be severe, and are very difficult to foresee.
Oceans will be hit hard as well. The most basic microscopic organisms such as plankton may not be able to survive. If that happened, it would mean that all of the other animals that are above plankton in the food chain would also die out. Other ecosystems such as forests and deserts will also be harmed.
The planet’s climate could also be affected by depletion of the ozone layer. Wind patterns could change, resulting in climatic changes throughout the world.
Essay # 6. Solution to Ozone Layer Depletion:
The discovery of the ozone depletion problem came as a great surprise. Now, action must be taken to ensure that the ozone layer is not destroyed.
Because CFCs are so widespread and used in such a great variety of products, limiting their use is hard. Also, since many products already contain components that use CFCs, it would be difficult if not impossible to eliminate those CFCs already in existence.
The CFC problem may be hard to solve because there are already great quantities of CFCs in the environment. CFCs would remain in the stratosphere for another 100 years even if none were ever produced again.
Despite the difficulties, international action has been taken to limit CFCs. In the Montreal Protocol, 30 nations worldwide agreed to reduce usage of CFCs and encouraged other countries to do so as well.
However, many environmentalists felt the treaty did “too little, too late”, as the Natural Resources Defense Council put it. The treaty asked for CFC makers to only eliminate half of their CFC production, making some people feel it was inadequate.
By the year 2000, the US and twelve nations in Europe have agreed to ban all use and production of CFCs. This will be highly significant, because these countries produce three quarters of the CFCs in the world.
Many other countries have signed treaties and written laws restricting the use of CFCs. Companies are finding substitutes for CFCs, and people in general are becoming more aware of the dangers of ozone depletion.