Are you looking for an essay on the ‘Plant Tissue Culture’? Find paragraphs, long and short essays on the ‘Plant Tissue Culture’ especially written for school and college students.
Essay on Plant Tissue Culture
Essay Contents:
- Essay on the Introduction to Plant Tissue Culture
- Essay on the Laboratory Requirements for Plant Tissue Culture
- Essay on the Components of Culture Medium
- Essay on the Techniques of Plant Tissue Culture
- Essay on the Types of Plant Tissue Culture
- Essay on the Protoplast Culture
- Essay on the Somaclonal Variation
1. Essay on the Introduction to Plant Tissue Culture:
Plant tissue culture is the method of culturing plant parts in an artificial medium to regenerate into a new plant. In horticulture, it is applied for the commercial production of disease and pest free plants on a commercial scale. It is a fascinating and useful tool which allows the rapid production of many genetically identical plants using relatively small amounts of space, supplies and time. Micro-propagation is the rapid vegetative propagation of plants via tissue culture techniques.
It permits the manipulation of physical and chemical conditions in the production of large numbers of high quality plant material within a short period of time. Basically the technique consists of taking a piece of a plant (such as a stem tip, node, meristem, embryo, or even a seed) and placing it in a sterile, (usually gel-based) nutrient medium supplemented with growth hormones, where it multiplies. The formulation of the growth medium can be varied according to the need of culture like undifferentiated callus tissue, multiply the number of plantlets, rooting, or multiply embryos for “artificial seed”.
The development of plant tissue began with the observation of French botanist George Morel (1965), while he was attempting to obtain a virus-free orchid plant and found that a millimetre-long shoot could be developed into complete plantlets by micropropagation. Thereafter, in the 1970s developed countries began commercial exploitation of this technology. It entered the developing world in the 1980s. It was earlier used to develop ornamental plants and flowering plants for export.
The in vitro techniques were developed initially to demonstrate the totipotency of plant cells predicted by Haberlandt in 1902. Totipotency is the ability of a plant cell to perform all the functions of development, which are characteristic of zygote, i.e., ability to develop into a complete plant. Haberlandt reported that culture of isolated single palisade cell from a leaf remained alive for up to 1 month, increased in size, accumulated starch but failed to divide in Knop’s salt solution enriched with sucrose. Efforts to demonstrate totipotency led to the development of techniques for cultivation of plant cells under defined conditions. Most of the modern tissue culture media derive from the work of Skoog and coworkers during 1950s and 1960s.
A successful establishment of callus culture depended on the discovery during mid- thirties of IAA (idole-3-acetic acid), the endogenous auxin, and of the role of vitamins in plant growth and in root cultures. The first continuously growing callus cultures were established by Gautheret, White and Nobecourt in 1939 from cambium tissue. The subsequent discovery of kinetin by Miller and co-workers in 1955 enabled the initiation of callus cultures from differentiated tissues. Shoot bud differentiation from tobacco pith tissues cultured in vitro was reported by Skoog in 1944, and in 1957 Skoog and Miller proposed that root-shoot differentiation in this system, which was regulated by auxin-cytokinin ratio.
In 1972, Carlson and coworkers produced the first somatic hybrid plant by fusing the protoplasts of Nicotiana glauca and N. langsdorfii. Plant protoplasts are naked cells from which cell wall has been removed. In 1960, Cocking produced large quantities of protoplasts by using cell wall degrading enzymes. The techniques of protoplast production have now been considerably refined and are now possible to regenerate whole plants from protoplasts and also to fuse protoplasts of different plant species. Since then many divergent somatic hybrids have been produced.
The first embryo culture, although crude, was done by Hanning in 1904; he cultured nearly mature embryos of certain crucifers and grew them to maturity. Haploid plants from pollen grains were first produced by Maheshwari and Guha in 1964 by culturing anthers of Datura. This marked the beginning of anther culture or pollen culture for the production of haploid plants.
Tissue culture cells generally lack the distinctive features of most plant cells. They have a small vacuole, lack chloroplasts and photosynthetic pathways and the structural or chemical features that distinguish so many cell types, within the intact plant are found to be absent. They are most similar to the undifferentiated cells found in meristematic regions which become fated to develop into each cell type as the plant grows.
Tissue cultured cells can also be induced to re-differentiate into whole plants by alterations to the growth media. Plant tissue cultures can be initiated from almost any part of a plant. The source, termed explant, must be healthy and free from obvious signs of disease or decay. Younger tissue contains a higher proportion of actively dividing cells (meristamatic) and is more responsive to a callus initiation programme. The exact conditions required to initiate and sustain plant cells in culture, or to regenerate intact plants from cultured cells, are different for each plant species.
2. Essay on the Laboratory Requirements for Plant Tissue Culture:
The essential requirements for proper tissue culture are analytical balance (for weighing nutrients for media), graduated cylinders and pipettes (for measuring stock solutions), pH meter (to regulate pH of media), microwave oven (to heat and dissolve gelling agent), glass containers (for heating and dissolving media), dispensing devices (to dispense equal quantities of media), de-ionizer (water needed for media), autoclave (for sterilizing instruments and media), transfer instruments (forceps, scalpels spatulas, blades), refrigerator (storage of chemicals and stock solutions), stereo-microscope (use for meristem culture) and laminar flow hood (provide a sterile area for transfers during initiation and sub-culturing).
3. Essay on the Components of Culture Medium:
One of the most important factors governing the growth and morphogenesis of plant tissues in culture is the composition of the culture medium. The basic nutrient requirements of cultured plant cells are very similar to those of whole plants.
Plant tissue and cell culture media are generally made up of some or all of the following components – Macronutrients, Micronutrients, Carbon and energy source, Vitamins, Amino acids or other nitrogen supplements, sugar(s), Other undefined organic supplements, Solidifying agents or support systems, and Growth regulators.
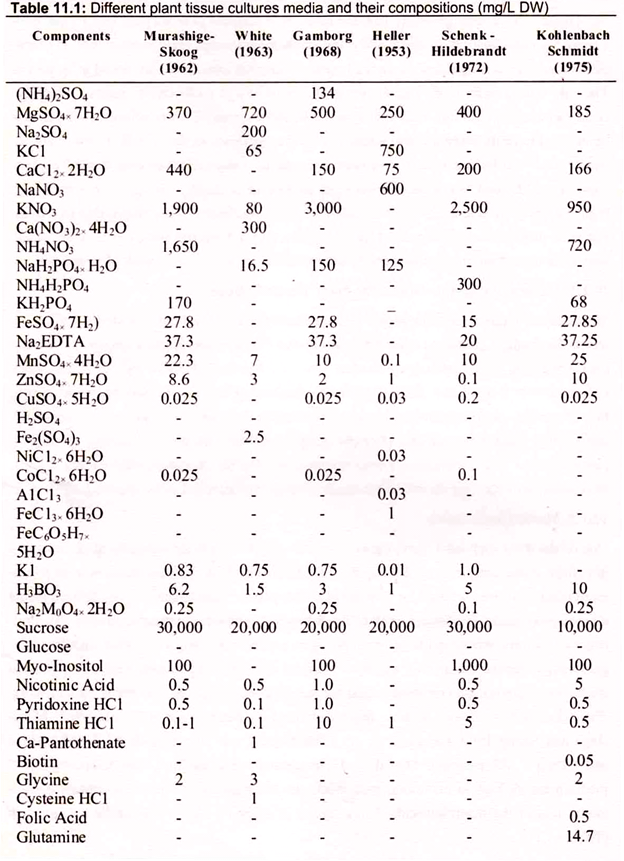
Several media formulations are commonly used for the majority of all cell and tissue culture work. These media formulations include those described by many workers. Murashige and Skoog’s MS medium, Schenk and Hildebrand’s SH medium, and Gamborg’s B-5 medium are all high in macronutrients, while the other media formulations contain considerably less of the macronutrients. Composition of some of the general media are given in (Table 11.1).
Plants grown in vitro require similar nutrients present in their natural environment.
The basic components of any cultural medium are:
(a) Macronutrients:
These elements are required in large amounts for plant growth and development. The macronutrients provide the six major elements-nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S)-required for plant cell or tissue growth. The optimum concentration of each nutrient for achieving maximum growth rates varies considerably among species. Culture media should contain at least 25-60 mM of inorganic nitrogen for adequate plant cell growth. Plant cells may grow on nitrates alone, but considerably better results are obtained when the medium contains both a nitrate and ammonium nitrogen source. Certain species require ammonium or another source of reduced nitrogen for cell growth to occur.
Nitrates are usually supplied in the range of 20-25 mM; typical ammonium concentrations range between 2 to 20 mM, however, excess of 8 mM may be deleterious to cell growth of certain species. Cells can grow on a culture medium containing ammonium as the sole nitrogen source if one or more of the TCA cycle acids (e.g., citrate, succinate, or malate) are also included in the culture medium at concentrations of approximately 10 mM. Potassium is required for cell growth of most plant species. Most media contain potassium, in the nitrate or chloride form, at concentrations of 20-30 mM. The optimum concentrations of P, Mg, S, and Ca range from 1-3 mM provide all other requirements.
(b) Micronutrients:
These elements are required in trace amounts for plant growth and development. The essential micronutrients for plant cell and tissue growth include iron (Fe), manganese (Mn), zinc (Zn), boron (B), copper (Cu), and molybdenum (Mo). Chelated forms of iron and zinc are commonly used in preparing culture media. Iron may be the most critical of all the micronutrients. Iron citrate and tartrate may be used in culture media, but these compounds are difficult to dissolve and frequently precipitate after media are prepared.
Murashige and Skoog used an ethylene diamine tetraacetic acid (EDTA)-iron chelate to bypass this problem. Cobalt (Co) and iodine (I) may also be added to certain media, but strict cell growth requirements for these elements have not been established. Sodium (Na) and chlorine (CI) are also used in some media but are not essential for cell growth. Copper and Cobalt are normally added to culture media at concentrations of 0.1 µM, Fe and Mo at 1 µM, I at 5 µM, Zn at 5- 30 µM, Mn at 20-90 µM, and B at 25-100 µM.
(c) Carbon and Energy Source:
Sucrose is the most commonly used carbohydrate source in plant cell culture media, though glucose and fructose can be substituted in some cases. Other carbohydrates that have been tested include lactose, galactose, rafinose, maltose, and starch. Sucrose concentrations of culture media normally range between 2 to 3 percent. Carbohydrates must be supplied to the culture medium because few plant cell lines have been isolated that are fully autotropic, e.g., capable of supplying their own carbohydrate by CO2 assimilation during photosynthesis.
(d) Vitamins:
Normal plants synthesize the vitamins required for their growth and development. Vitamins are required by plants as catalysts in various metabolic processes. When plant cells and tissues are grown in vitro, some vitamins may become limiting factors for cell growth. The vitamins most frequently used in cell and tissue culture media include thiamin (B1), nicotinic acid, pyridoxine (B6), and myo-inositol. Thiamin is the one vitamin that is basically required by all cells for growth. Thiamin is normally used at concentrations ranging from 0.1 to 10.0 mg/liter. Nicotinic acid and pyridoxine are often added to culture media but are not essential for cell growth in many species.
Nicotinic acid is normally used at concentrations of 0.1-5.0 mg/liter; pyridoxine is used at 0.1-10.0 mg/liter. Afyo-inositol is commonly included in many vitamin stock solutions. Although it is a carbohydrate not a vitamin, it has been shown to stimulate growth in certain cell cultures. Its presence in the culture medium is not essential, but in small quantities myo-inositol stimulates cell growth in most species. It is generally used at concentrations of 50-5000 mg/liter.
Other vitamins such as biotin, folic acid, ascorbic acid, pantothenic acid, vitamin E (tocopherol), riboflavin, and p-aminobenzoic acid have been included in some cell culture media. The requirement for these vitamins by plant cell cultures is generally negligible, and they are not considered growth-limiting factors. These vitamins are generally added to the culture medium only when the concentration of thiamin is below the desired level or when it is desirable to grow cells at very low population densities.
(e) Amino Acids or Other Nitrogen Supplements:
Certain amino acids or amino acid mixtures are found to stimulate cell growth though cultured cells are normally capable of synthesizing all of the required amino acids. The use of amino acids is particularly important for establishing cell cultures and protoplast cultures. Amino acids provide plant cells with an immediately available source of nitrogen, which generally can be taken up by the cells more rapidly than inorganic nitrogen. The most common sources of organic nitrogen used in culture media are amino acid mixtures (e.g., casein hydrolysate), L-glutamine, L-asparagine, and adenine.
Casein hydrolysate is generally used at concentrations between 0.05 and 0.1 percent. When amino acids are added alone, care must be taken, as they can be inhibitory to cell growth. Examples of amino acids included in culture media to enhance cell growth are glycine at 2 mg/liter, glutamine up to 8 mM, asparagine at 100 mg/liter, L-arginine and cysteine at 10 mg/liter, and L-tyrosine at 100 mg/ liter. Tyrosine has been used to stimulate morphogenesis in cell cultures but should only be used in an agar medium. Supplementation of the culture medium with adenine sulfate can stimulate cell growth and greatly enhance shoot formation.
(f) Organic Supplements:
Addition of a wide variety of organic extracts to culture media often results in favorable tissue responses. Supplements that have been tested include protein hydrolysates, coconut milk, yeast extracts, malt extracts, ground banana, orange juice and tomato juice. However, undefined organic supplements should only be used as a last resort, and only coconut milk and protein hydrolysates are used to any extent today. Protein (casein) hydrolysates are generally added to culture media at a concentration of 0.05-0.1%, while coconut milk is commonly used at 5-20% (v/v).
The addition of activated charcoal (AC) to culture media may have a beneficial effect. The effect of AC is generally attributed to one of three factors: absorption of inhibitory compounds, absorption of growth regulators from the culture medium, or darkening of the medium. The inhibition of growth in the presence of AC is generally attributed to the absorption of phytohormones to AC. 1-Napthaleneacetic acid (NAA), kinetin, 6-enzylamino- purine (BA), indole-3-acetic acid (IAA), and 6-α-α-dimethylallylaminopurine (2iP) all bind to AC, with the latter two growth regulators binding quite rapidly. The stimulation of cell growth by AC is generally attributed to its ability to bind to toxic phenolic compounds produced during culture. Activated charcoal is generally acid-washed prior to addition to the culture medium at a concentration of 0.5-3.0 percent.
(g) Solidifying Agents or Support Systems:
Agar is the most commonly used gelling agent for preparing semisolid and solid plant tissue culture media. Agar has several advantages over other gelling agents. First, when agar is mixed with water, it forms a gel that melts at approximately 60-100 °C and solidifies at approximately 45°C; thus, agar gels are stable at all feasible incubation temperatures.
Additionally, agar gels do not react with media constituents and are not digested by plant enzymes. The firmness of an agar gel is controlled by the concentration and brand of agar used in the culture medium and the pH of the medium. The agar concentrations commonly used in plant cell culture media range between 0.5 and 1.0%; these concentrations give a firm gel at the pH typical of plant cell culture media.
(h) Growth Regulators:
Auxins, cytokinins, gibberellins, and abscisic acid are the four important classes of growth regulators in plant tissue culture. Skoog and Miller (1957) were the first to report that the ration of auxin to cytokinin determined the type and extent of organogenesis in plant cell cultures. Both, an auxin and cytokinin are usually added to culture media in order to obtain morphogenesis, although the ratio of hormones required for root and shoot induction is not universally the same.
Considerable variability exists among genera, species, and even cultivars in the type and amount of auxin and cytokinin required for induction of morphogenesis. The auxins commonly used in plant tissue culture media are 1H-indole-3-acetic acid (IAA), 1H-indole-3-butyric acid (IBA), (2, 4-dichlorophenoxy) acetic acid (2,4-D), and 1- napthaleneacetic acid (NAA). The only naturally occurring auxin found in plant tissues is IAA. Other synthetic auxins that have been used in plant cell culture include 4- chlorophenoxyacetic acid or p-chlorophenoxyacetic acid (4-CPA, PCPA), (2,4,5- trichlorophenoxy) acetic acid (2,4,5-T), 3,6- dichloro-2-methoxybenzoic acid and 4-amino- 3,5,6-trichloropicolinic acid.
The cytokinins commonly used in the culture media include 6-benzylaminopurine or 6- benzyladenine (BAP, BA), N-(2-furanylmethyl)-1H-puring-6-amine (kinetin), and 6-(4- hydroxy-3-methyl-trans-2-butenylamino) purine (zeatin). Zeatin is considered to be naturally occurring cytokinin, while BAP and kinetin are synthetically derived cytokinins. The cytokinins are generally added to a culture medium to stimulate cell division, to induce shoot formation and axillary shoot proliferation, and to inhibit root formation.
The type of morphogenesis that occurs in a plant tissue culture largely depends upon the ratio and concentrations of auxins and cytokinins present in the medium. Root initiation of plantlets, embryogenesis, and callus initiation all generally occur when the ratio of auxin to cytokinin is high, whereas adventitious and axillary shoot proliferation occurs when the ration is low. Gibberellins (GA3) and abscisic acid (ABA) are two other growth regulators occasionally used in culture media.
Plant tissue cultures can usually be induced to grow without either GA3 or ABA, although, certain species may require these hormones for enhanced growth. Generally, GA3 is added to culture media to promote the growth of low-density cell cultures, to enhance callus growth, and to elongate dwarfed or stunted plantlets. Abscisic acid is generally added to culture media to either inhibit or stimulate callus growth (depending upon the species), to enhance, inhibit, or stimulate callus growth (depending upon the species), to enhance shoot or bud proliferation, and to inhibit latter stages of embryo development.
4. Essay on the Techniques of Plant Tissue Culture:
In plant tissue culture, explants such as pieces of leave, stem or root is cultured in a specific plant medium, which contains essential plant nutrients and hormones. The explants are surface sterilized by using chemical solutions such as bleach or alcohol. Though, mercury chloride is an effective sterilizer, it is rarely used due to its potential toxicity. After sterilization, the explants are introduced into a plant medium, which can be either solid or liquid.
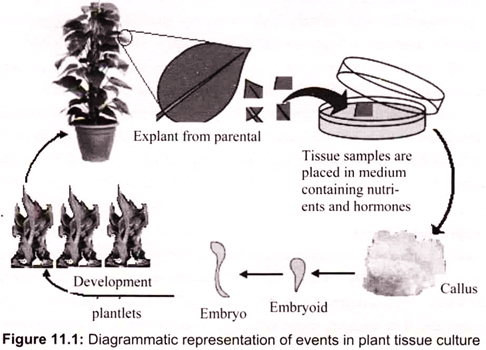
Other plant growth factors like light and temperature are maintained and regulated by using artificial conditions. All the procedures of plant tissue culture are conducted under sterile (aseptic) conditions. Generally, these give rise to an unorganized mass of cells called callus (soft tissue that forms over a cut surface). The cells divide and differentiate into plant parts, thus giving rise to a complete plant (Fig. 11.1). The explants then develop stem, roots and leaves.
The generated plantlets are hardened before planting in outdoor conditions. In addition to these ingredients, solid medium contains gelling agent (agar). Nutrient and plant hormones amount vary, depending on the objective of plant tissue culture. For example, in order to induce more roots, auxin amount should be high. Plant tissue culture techniques include the culture of protoplast (a cell without cell wall), meristem, node, anther, ovule, embryo and seed.
5. Essay on the Types of Plant Tissue Culture:
Cultures are generally initiated from sterile pieces of a whole plant, termed explants, and may consist of pieces of organs, such as leaves or roots, or may be specific cell types, such as pollen or endosperm. Many features of the explant are known to affect the efficiency of culture initiation.
Generally, younger, more rapidly growing tissue (or tissue at an early stage of development) is most effective:
a. Callus:
Formation of callus, an unorganized, growing, and dividing mass of cells, can be induced on explants when they are cultured on an appropriate medium, usually with a supplementation of both auxin and cytokinin. In tissue culture, proliferation of callus can be maintained more or less indefinitely, provided that the callus is sub-cultured on to fresh medium periodically. During callus formation, there can be some degree of dedifferentiation (i.e. the changes that occur during development and specialization are, to some extent, reversed), both in morphology (a callus is usually composed of unspecialized parenchyma cells) and metabolism.
One major consequence of this dedifferentiation is that most plant cultures lose the ability to photosynthesize. This has important consequences for the culture of callus tissue, as the metabolic profile will probably not match with that of the donor plant. This necessitates the addition of other components—such as vitamins and, most importantly, a carbon source—to the culture medium, in addition to the usual mineral nutrients.
Callus culture is often performed in the dark (the lack of photosynthetic capability being no drawback) as light can encourage differentiation of the callus. During long-term culture, the culture may lose the requirement for auxin and/or cytokinin. This process, known as habituation, is common in callus cultures from some plant species (such as sugar beet). Callus cultures, broadly speaking, fall into one of two categories: compact or friable. In compact callus, the cells are densely aggregated, whereas in friable callus, the cells are only loosely associated with each other and the callus becomes soft and breaks apart easily.
Callus cultures are extremely important in plant biotechnology. Manipulation of the auxin to cytokinin ratio in the medium can lead to the development of shoots, roots, or somatic embryos from which whole plants can subsequently be produced. It can also be used to initiate cell suspensions, which are used in a variety of ways in plant transformation studies.
b. Cell-Suspension Cultures:
In suspension culture, cells are suspended in the medium rather than adhering to a surface. Friable callus provides the inoculum to form cell-suspension cultures. When friable callus is placed into a liquid medium (usually the same composition as the solid medium used for the callus culture) and then agitated, single cells and/or small clumps of cells are released into the medium.
Under the proper conditions, these released cells continue to grow and divide, eventually producing a cell-suspension culture. A relatively large inoculum should be used when initiating cell suspensions so that the released cell numbers build up quickly. The inoculum should not be too large though, as toxic products released from damaged or stressed cells can build up to lethal levels. Large cell clumps can be removed during subculture of the cell suspension.
Explants from some plant species or particular cell types tend not to form friable callus, making it difficult to initiate cell suspension. The friability of the callus can sometimes be improved by manipulating the medium components or by repeated sub-culturing. The friability of the callus can also sometimes be improved by culturing it on semi-solid medium (medium with a low concentration of gelling agent).
Cell suspensions can be maintained relatively simply as batch cultures in conical flasks. They are continually cultured by repeated sub-culturing into fresh medium. This results in dilution of the suspension and the initiation of another batch growth cycle. The degree of dilution during subculture should be determined empirically for each culture. A high-degree of dilution will result in a greatly extended lag period or, in extreme cases, death of the transferred cells.
After subculture, the cells divide and the biomass of the culture increases in a characteristic fashion, until nutrients in the medium are exhausted and/or toxic by-products build up to inhibitory levels, called the stationary phase. If cells are left in the stationary phase for too long, they will die and the culture will be lost. Therefore, cells should be transferred as they enter the stationary phase. It is therefore important that the batch growth-cycle parameters are determined for each cell-suspension culture.
c. Root Cultures:
Root cultures can be established in vitro from explants of the root tip of either primary or lateral roots and can be cultured on fairly simple media. The growth of roots in vitro is potentially unlimited, as roots are indeterminate organs. Although, the establishment of root cultures was one of the first achievements of modern plant tissue culture, they are not widely used in plant transformation studies.
d. Anther Culture:
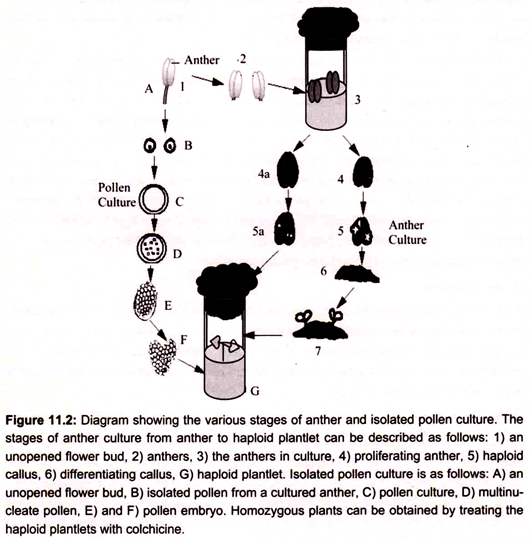
Anther culture is the process of using anthers to culture haploid plantlets. The technique was discovered in 1964 by Guha and Maheshwari. This technique can be used in over 200 species, including tomato, rice, tobacco, barley, and geranium. Some of the advantages which make this a valuable method for obtaining haploid plants. It is easy to induce cell division in the immature pollen cells in some species, high induction frequency and large number of haploids can be produced in a short period of time. For example in case of Datura innoxia, induction frequencies of almost 100% and a yield of more than one thousand plantlets or calluses have occurred under optimal conditions from one anther. Success can be determined within 24 hours as cells begin to divide (Fig. 11.2).
Some of the disadvantages of using anther culture to obtain haploids involves, when working with some species, the majority of plants produced have been non-haploid. Particularly in certain cases like cereals, very few green plants are obtained; many of the plants are albinos or green-albino chimeras. Generally, it is tedious to remove the anthers without causing damage and sometimes a particular orientation is necessary to achieve a desired response.
e. Embryo Culture:
Embryos can be used as explants to generate callus cultures or somatic embryos. Both immature and mature embryos can be used as explants. Immature, embryo-derived embryogenic callus is the most popular method of monocotyledon plant regeneration.
Somatic Embryogenesis:
In somatic (asexual) embryogenesis, embryo-like structures, which can develop into whole plants in a way analogous to zygotic embryos, are formed from somatic tissues. These somatic embryos can be produced either directly or indirectly. In direct somatic embryogenesis, the embryo is formed directly from a cell or small group of cells without the production of an intervening callus. Though, common from some tissues (usually reproductive tissues such as the nucellus, styles, or pollen), direct somatic embryogenesis is generally rare in comparison with indirect somatic embryogenesis.
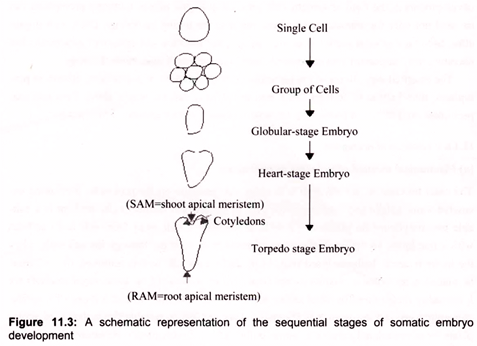
Somatic embryos may develop from single cells or from a small group of cells (Fig. 11.3). Repeated cell divisions lead to the production of a group of cells that develop into an organized structure known as a globular-stage embryo. Further development results in heart- and torpedo-stage embryos, from which plants can be regenerated. Zygotic embryos undergo a fundamentally similar development through the globular (which is formed after the 16-cell stage), heart and torpedo stages. Polarity is established early in embryo development. Signs of tissue differentiation become apparent at the globular stage and apical meristems are apparent in heart-stage embryos. Embryogenesis is particularly favored for transformation work.
6. Essay on the Protoplast Culture:
Protoplast is the living material of a plant or bacterial cell, including the protoplasm and plasma membrane after the cell wall has been removed. E.C. Cocking in 1960 at the University of Nottingham (U.K) demonstrated that naked cells called protoplasts can be obtained through enzymatic degradation of cell walls. Protoplast isolation, fusion and development of fused protoplast in to a complete plant are one of the most significant developments in the field of plant tissue culture, witnessed during the last few decades.
This led to significant developments in the field of somatic cell genetics in higher plants. Cultured protoplasts can be used not only for somatic cell fusions, but also for taking up foreign DNA, cell organelles, bacteria and virus particles. In view of this, the isolation and culture of protoplasts has become a very important area of research, within the realm of plant biotechnology.
The essential ingredients of the technique include isolation of protoplasts, culture of protoplasts, introduction of foreign DNA into protoplasts, raising whole plants from cultured protoplasts and fusion of protoplasts leading to somatic hybridization.
I. Protoplast Culture and Regeneration of Plants:
The culture methods for isolated protoplasts are similar to those used for single cells. Both semi-solid and liquid medium can be used, although the liquid medium is preferred. Protoplasts can be suspended in a liquid medium in Erlenmeyer flasks without shaking and can be cultured in small quantities in ‘hanging drops’ or in ‘microchambers’. Within 2-4 days, protoplasts start developing cell walls, which can be detected by staining with 0.1% calcofluor white (CPW) fluorescent stain, while the presence of a proper wall is essential for a regular division.
The protoplasts, which are capable of dividing, undergo first division within 2-7 days and form multicellular colonies after 2- 3 weeks. After another two weeks, these colonies can be treated as standard tissue cultures. From these colonies or tissues in culture, plants can be regenerated. The regeneration technique involves transfer of callus to a medium capable of initiating differentiation and it behaves just like the callus derived from cells. Shooting and rooting can be induced by manipulating the hormone concentration. Subsequently, the generated plantlets may be transferred to pots or field after hardening.
II. Methods for Protoplast Isolation:
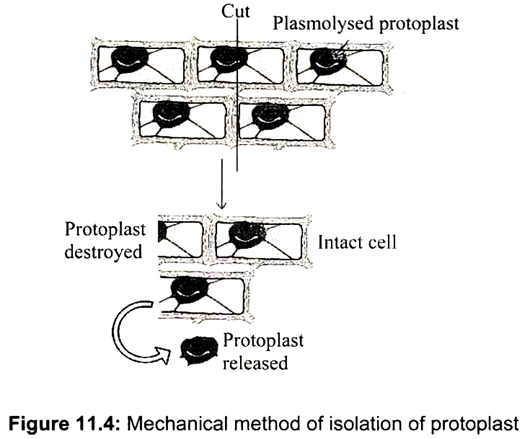
a. Mechanical Method of Isolation of Protoplast:
The chief function of the cell wall is to exert wall pressure on the protoplast preventing excessive water uptake and bursting of the cell. In mechanical method, cells are kept in a suitable plasmolyticum (in plasmolysed cells, protoplasts shrink away from cell wall) and cut with a fine knife, so that protoplasts are released from cells cut through the cell wall, when the tissue is again deplasmolysed (Fig. 11.4). Before the cell wall is removed, the cell must be placed in an isotonic plasmolyticum (mannitol or sorbitol 13 %, these sugar alcohols are less readily metabolised by plant cells).
It may be advantageous to test a range of mannitol concentrations varying from 8-15% (w/v). This method is suitable for isolation of protoplasts from vacuolated cells (e.g. onion bulbs, scales, radish roots). However, this method gives poor yield of protoplasts and is not suitable for isolating protoplst from meristematic and less vacuolated cells. The mechanical method, though, was used as early as 1892, is now only rarely used for isolation of protoplasts. One advantage, however, is that the deleterious effects of the wall-degrading enzymes on the metabolism of the protoplasts are eliminated.
b. Enzymatic Method of Isolation of Protoplast:
The enzymatic method has the advantage that it gives large quantities of protoplasts, where cells are not broken and osmotic shrinkage is minimum. But for better results sometimes mechanical and enzymatic methods are combined, where cells are first separated mechanically and later used for isolation of protoplasts through enzymatic treatment. The protoplasts can be isolated from a variety of tissues including leaves, roots, in vitro shoot cultures, callus, cell suspension and pollen. Young cell suspensions are particularly ideal for isolation of protoplasts in large quantities.
Protoplast isolation is achieved by using cellulase in combination with pectinase and hemicellulase. Commercial preparations of enzymes are derived from microorganisms, and may contain ribonucleases, proteases, and several other toxic enzymes. As a result of these deleterious enzymes, several purification procedures have been developed (one of the most often used procedures is column separation).
There are two approaches to the use of wall- degrading enzymes:
i. Mixed-enzyme method- pectinase and cellulase and/or hemicellulase are applied simultaneously.
ii. Sequential method- involves treatment with pectinase (loosen the cells), followed by cellulase and or hemicellulase.
The major steps employed for isolation of protoplasts involve:
(i) Sterilization of leaves,
(ii) Peeling off the epidermis,
(iii) Enzymatic treatment and
(iv) Isolation and cleaning of protoplasts.
For protoplast isolation the explants are generally sterilized by first dipping them into 70% ethyl alcohol for about a minute and then treating them with 2% solution of sodium hypochlorite for 20-30 minutes. They are then rinsed three times with sterile distilled water and subsequent operations are carried out under aseptic conditions. In case of leaves the lower epidermis of the sterilized leaves is carefully peeled off and the stripped leaves are cut into small pieces.
Mesophyll protoplasts can be obtained from these peeled leaf segments.
In case of cereals, where it is difficult to peel off the epidermis, leaves are cut in long strips and used with enzyme mixture in either of the way as discussed above, such as:
(i) Direct (one step) method, in which treatment with macerozyme (or pectinase) and cellulase is done simultaneously, here the enzyme mixture consists of 0.5% macerozyme + 2% cellulase in 13% sorbitol or mannitol at pH 5.4, or
(ii) Sequential (two step) method, in which cells are first isolated using macerozyme and cells are then treated with cellulase to isolate protoplasts.
III. Protoplast Fusion and Its Types:
Protoplast fusion or somatic hybridization is one of the most important uses of protoplast culture. This is particularly significant for hybridization between species or genera, which cannot be made to cross by conventional method of sexual hybridization. Although somatic hybridization was successfully achieved first in animals and later in plants, its significance has been realized fully in plants because the hybrid cells can be induced to regenerate into whole plants.
a. Spontaneous Protoplast Fusion:
The spontaneous fusion of protoplast is found to be strictly intra-specific. However, spontaneous fusion of protoplasts can also be induced by bringing protoplasts into intimate contact through micromanipulators or micropipettes. There seems to be a correlation between the size of the leaf and the percentage of protoplasts undergoing spontaneous fusion; protoplasts from young leaves are more likely to undergo this fusion.
b. Induced Protoplast Fusion:
Somatic hybridization is generally used for fusion of protoplasts either from two different species (interspecific fusion) or from two diverse sources belonging to the same species. In plants, however, the inducing agent (fusogen) first brings the protoplasts together and then causes them to adhere to one another for bringing about fusion. During the last two decades, a variety of treatments have been successfully utilized for fusion of plant protoplasts. It involves the treatments particularly with NaNO3, high pH with high Ca++ ion concentration and polyethylene glycol (PEG).
(i) NaNO3 Treatment:
The method involves two steps such as isolated protoplasts are suspended in an aggregation mixture (5.5% sodium nitrate in 10% sucrose solution). This mixture works as a fusion inducing mixture and causes fusion on incubation at 35°C. In order to obtain a higher frequency of fused protoplasts, the mixture may be centrifuged and the pellet re-suspended and incubated for one or more additional cycles. Finally the mixture is replaced by a liquid medium and the protoplasts in this mixture are incubated again; the cycle may be repeated once or twice before plating the protoplasts on a solid medium. The fusion of protoplasts can be monitored at different steps by examination under an inverted microscope.
This method was successfully utilized for fusion of protoplasts from root tips of oat and maize seedlings but is not preferred due to low frequency of fusion, particularly when highly vacuolated mesophyll protoplasts are used.
(ii) Treatment with Calcium Ions (Ca++) at High pH:
This method involves spinning (centrifugation) the protoplasts in a fusion inducing solution (0.05M CaCl2.2H2O) in 4M mannitol at pH 10 for 30 min at 50g, after which the tubes are placed in a water bath (37o) for 40-50 min. This leads to fusion of 20-50% of the protoplasts. A calcium solution buffered at high pH induces aggregation of the protoplasts and their fusion. The addition of Ca++ causes the potential of the surface negative charge on protoplasts to be reduced, facilitating protoplast adhesion. The high alkalinity (pH 9.5-10.4) induces the formation of intra-membranous lysophospholipids such as lysolecithin and lysophosphatidyl -ethanolamine that increase membrane fluidity, which results in fusion. This method has been successfully used in the production of numerous somatic hybrid plants.
(iii) Polyethylene Glycol (PEG) Treatment:
PEG has been most widely used to fuse plant protoplasts. Both, the concentration and molecular weights of PEG are important in relation to fusion. PEG (HOCH2-(CH2-O-CH2) – CH2OH) is a water soluble compound whose linkages make the molecule slightly negative in charge. Thus, addition of Ca++ (CaCl2 or Ca (NO3)2 links the compound with membrane surfaces. The high molecular weight of the polymer acts as a bridge connecting the protoplasts together.
A strong affinity of PEG for water causes local membrane dehydration and increased fluidity. This in combination with the reduction of an exclusion volume between adjacent protoplasts causes diminishing mutual membrane electrostatic repulsion. The redistribution of glycoprotein and glycocalyx macromolecules causes fusion. Compounds structurally related to PEG, namely, polyvinyl alcohol, polyvinyl pyrrolidone and polyglycerol are known to induce fusion. Gelatin and dextran sulphate also induce fusion to varying degrees.
The technique gives high frequency of fusion with reproducible results and involves low cytotoxicity. The technique can be used for fusion of protoplasts from unrelated plant taxa (e.g. soybean -tobacco, soybean, maize, and soybean – barley), from unrelated animal taxa and also between those from animal and plant cells. PEG has been used in combination with other treatments to enhance fusion frequency. An increase in the frequency of heterokaryon formation was observed when protoplasts were pre-incubated in lysozyme. Also, combination of PEG with high pH Ca++ solution, or the addition of DMSO or concanavalin A gave rise to higher fusion frequencies in comparison to treatments with PEG alone. After PEG treatment, protoplasts are gradually washed and during this process, most of the fusion is achieved. PEG is then replaced by culture medium to allow growth of fused protoplasts.
(iv) Electrical Fusion:
Protoplasts can be induced to fuse by placing them into a small culture cell containing electrodes and a potential difference is applied, then the protoplasts will line up between the electrodes. Application of an extremely short, square, wave of electric shock will now induce them to fuse.
After the fusion treatment the protoplast population consists of a mixture of parental types, homokaryons and heterokaryons of which heterokaryons (potential source of future hybrids) often make only 0. 5%-10%.
IV. Somatic Hybridization and Its Applications:
Production of hybrid plants through the fusion of protoplasts of two different plant species/ varieties is called somatic hybridization and such hybrids are known as somatic hybrids. Protoplast technology, which includes the isolation, culture, and fusion of higher plant protoplasts leading to the production of whole plants, is considered one of the most exciting developments in experimental botany in recent years. Protoplast culture provides excellent opportunities for research on plant improvement, first by exploring genetic variations among the existing crops and then by attempting to transfer the available genetic information from one species to another through fusion of protoplasts isolated from somatic tissues of these crops.
Somatic hybridization involves fusion of two distantly related, to closely related plant protoplasts at intraspecific, interspecific, intergeneric, and interfamily levels, with subsequent regeneration of hybrid cells into hybrid plants. Plastids and mitochondrial genomes (cytoplasmically encoded traits) are inherited maternally in sexual crossings. Through the fusion process, the nucleus and cytoplasm of both parents are mixed in the hybrid cell (heterokaryon). This results in various nucleocytoplasmic combinations. Sometimes interactions in the plastome and genome contribute to the formation of cybrids (cytoplasmid hybrids).
Conventional sexual crossing in higher plants is a highly regulated system of hybridization wherein sexual crosses are limited to phylogenetically related plant species. Also, the classical methods of breeding employed for transfer of beneficial traits from wild species to cultivated varieties are time consuming and require extensive backcrossing with the cultivated variety in order to eliminate most of the genome of the wild species while retaining the useful genes.
V. Cytoplasmic Hybrids for Cybrids:
Cybrids, in contrast to conventional hybrids, possess a nuclear genome from only one parent but cytoplasmic genes, from both parents. The process of protoplast fusion resulting in the development of cybrids is known as cybridization. In cybridization, heterozygosity of extra-chromosomal material can be obtained, which has direct application in plant breeding. The technique of cybrid production has been utilized for transfer of cytoplasmic male sterility as has been successfully done in Nicotiana, Brassica and Petunia. Other characters like streptomycin resistance have also been transferred from N. tobacum to N. sylvestris, using this technique. Ogura cytoplasmic-male sterility (CMS) lines with ‘Ogura cytoplasm’, an herbicide (atrazine) resistant line and lines with increased nectar production have also been obtained using cybrids in crop Brassica.
Cybrids can be obtained by methods such as fusion of normal protoplasts from one parent with enucleated protoplasts from the other parent; enucleated protoplasts can be obtained by high speed centrifugation (20,000 – 40,000g for 45-90 min) of protoplasts or by irradiation treatment, fusion of normal protoplasts from one parent and protoplasts containing non-viable nuclei from the other, selective elimination of one of the nuclei from the heterokaryon or by selective elimination of chromosomes of one parent at a later stage after fusion of the nuclei.
Application of Somatic Hybridization and Cybridization:
i. Somatic cell fusion appears to be the only means through which two different parental genomes can be recombined among plants that cannot reproduce sexually (asexual or sterile).
ii. Protoplasts of sexually sterile (haploid, triploid, and aneuploid) plants can be fused to produce fertile diploids and polyploids.
iii. Somatic cell fusion overcomes sexual incompatibility barriers. In some cases somatic hybrids between two incompatible plants have also found application in industry or agriculture.
iv. Somatic cell fusion is useful in the study of cytoplasmic genes and their activities and this information can be applied in plant-breeding experiments.
Procedures for Selection of Somatic Hybrids:
Proper selection of the hybrid cells or fusion products after fusion treatment is necessary since the protoplast populations consist of a heterogeneous mixture of unfused parental types, homokaryons, and heterokaryons. This is because the fusion induced by various methods is random and uncontrolled. The low number of true hybrid cells formed may get lost in the population of actively dividing homokaryotic fusion products and unfused parental protoplasts. Hence, selective recovery of the few hybrid cells formed from the mixed population of regenerating protoplasts is a key factor in successful somatic hybridization.
The different selection procedures employed are:
a. Biochemical Selection of Somatic Hybrids:
This selection procedure was based on a prior knowledge of the differential growth characteristics and nutritional requirements of unfused and hybrid protoplasts isolated from the genetically different species. Protoplasts of the hybrid are able to grow on a defined medium in culture to form calli, whereas parental types will fail to develop into calli. This selection system has an advantage in that the requirement of a mutant as one of the fusion partners is totally eliminated.
b. Visual Selection of Somatic Hybrids:
In most of the somatic hybridization experiments selection procedures involve fusion of chlorophyll deficient (nongreen) protoplasts of one parent with the green protoplasts of the other parent (wild type), since this facilitates visual identification of heterokaryons at the light microscope level. Nongreen protoplasts are isolated from cultured cells, epidermal cells, or antibiotic induced albino plantlets. Even though the mechanical method of isolation of somatic fusion products is the most tedious procedure, it may be the most likely method for recovering osmotic hybrids in a variety of different plants, especially legumes, cereals, and tree species.
This approach suffers the draw back from the fact that it requires special culture media for each particular hybrid cell type to divide and form clusters. This is called the fishing method. Somatic hybrid callus has been similarly obtained in fusion between colorless protoplasts of Glycine max derived from cell cultures with the green mesophyll protoplasts of Nicotiana glauca.
c. Morphological Selection of Somatic Hybrids:
Melchers et al. (1978) adopted the method of selection of somatic hybrids based on their abnormal morphology in regenerating intergeneric somatic hybrids such as “pomatoes” and ‘topatoes’ which are the fusion products of protoplasts of tomato and potato.
The regenerated plants showed abnormal morphology and proved to be somatic hybrids by analysis of chromosomal and fraction-1-protein, the ribulosel, 5-bisphosphate carboxylase. Intermediate morphology of the callus also determined the intergeneric somatic hybrids between Vicia faba and Petunia hybrida.
d. Flow Cytometry and Sorting Selection of Somatic Hybrids:
Various laboratories are using techniques of flow cytometry and fluorescent activated cell sorting for analysis of plant protoplasts while maintaining their viability. These techniques have also been applied for sorting and selection of heterokaryons. The fused and unfused products are sorted in a “cell sorter” machine based on the presence or absence of fluorescence of both dyes in the fusion products. Galbraith et al. (1989) have described a universally applicable method for electronic sorting of heterokaryons formed by fusing the protoplasts of two parents labeled with different vital fluorescent dyes, such as rhodomine isothio- cyanate and floreceine isothiocyanate.
7. Essay on the Somaclonal Variation:
In plants genetic variation occurs at stages of development of the tissue culture process such as the variations occur as a part of mutation or as a part of clonal development have been termed as somaclonal variations. As these variations are not due to heredity, their transmissions to next generations are not possible. However, such variations combined with action of mutagens could result into ideal breeding material for crop improvement programme.
Somaclonal variations affect cytoplasmic genome as well as nuclear genome. These variations, when heritable are useful for improvement of crops such as resistance to insect pests and diseases, tolerance to environmental stress, male sterility etc.
Examples of somaclonal variations observed in crop species are:
i. Rust resistance in wheat.
ii. Resistance for late blight in potato.
iii. Shortening of harvest duration in sugar cane.
iv. Increased shelf life in tomato.
v. Tolerance to high temperature in wheat.
vi. Resistance for leaf hopper in rice.
vii. High protein content in potato.
Isolation of somaclonal variation:
Mutants for several traits can be far more easily isolated from cell cultures than from whole plant populations. Mutants can be effectively selected for disease resistance, improvement of nutritional quality, adaptation of plants to stress conditions, e.g., saline soils, low temperature, toxic metals (e.g., aluminium), and resistance to herbicides and to increase the biosynthesis of plant products used for medicinal or industrial purposes.
The various approaches for isolation of somaclonal variants can be categorized as screening and cell selection:
(i) Screening:
It involves the observation of a large number of cells or regenerated plants for the detection of variant individuals. This approach is the only feasible technique for the isolation of mutants for yield and yield traits. Screening has been profitably and widely employed for the isolation of cell clones that produce higher quantities of certain bio-chemicals.
(ii) Cell selection:
In the cell selection approach, a suitable selection pressure is applied, which permits the preferential survival/growth of variant cells only. Some examples of cell selection are selection of cells resistant to various toxins, herbicides, high salt concentration, etc. When the selection pressure allows only the mutant cells to survive or divide, it is called positive selection. On the other hand, in the case of negative selection, the wild type cells divide normally and, therefore, are killed by a counter selection agent.
These cells are subsequently rescued by removal of the counter election agent. Negative selection approach is utilized for the isolation of auxotrophic mutants.
The positive selection approach may be further subdivided into four categories:
(i) Direct selection,
(ii) Rescue method,
(iii) Stepwise selection and
(iv) Double selection.
In direct selection, the cells resistant to the selection pressure survive and divide to form colonies; the wild type cells are killed by the selection agent. This is the most common selection method which it is used for the isolation of cells resistant to toxins (produced by pathogens), herbicides, elevated salt concentration, antibiotics, amino acid analogues, etc.
In the rescue method, the wild type cells are killed by the selection agent, while the variant cells remain alive but, usually, do not divide due to the unfavorable environment. The selection agent is then removed to recover the variant cells. This approach has been used to recover low temperature and aluminium resistant variant cells.
The selection pressure, e.g., salt concentration, may be gradually increased from a relatively low level to the cytotoxic level; the resistant clones isolated at each stage are subjected to the higher selection pressure. Such a selection approach is called stepwise selection.
Somaclonal variation has the advantages such as it occurs in rather high frequencies, which is a great advantage over conventional methods. Some ‘new’ alleles or even ‘new’ mutations may be isolated, which were not available in the germplasm or through mutagenesis. The use of somaclonal variation may reduce the time required for the release of new variety as compared to mutation breeding. This is the only approach for the isolation of biochemical mutants, especially auxotrophic mutants, in plants. A very effective selection can be practiced at the cell level for several traits, e.g., disease resistance.
One major advantage of plant tissue culture is the production of disease and/or pest resistant varieties, thus indirectly increasing the crop yield. Commercially, it is used directly for the propagation of plants that are hard to propagate in natural conditions. Horticultural plants such as orchids, roses, banana, strawberries, potatoes, apples, etc. are successfully cultured in in-vitro conditions. Plants with valuable secondary products are grown in the controlled conditions by using plant tissue culture technique. It is also used for the propagation of medicinal herbs on a large scale. With plant tissue culture, it is possible to generate; virus-free plantlet of vegetatively propagated plants.
In experimental biology such as plant breeding, cell biology, biotechnology and genetics, plant tissue culture is applied in order to solve plant related problems. It allows screening of cells for the desirable characters such as early fruit bearing, disease resistance and drought resistance. Another important application is generation of a novel hybrid by crossing two distantly related species having advantageous traits. In-vitro fertilization and/or pollination of plants is possible, irrespective of the hindrances in natural conditions. In short plant tissue culture is one of the methods for conservation of germplasm.