Are you looking for an essay on the ‘Animal Tissue Culture’? Find paragraphs, long and short essays on the ‘Animal Tissue Culture’ especially written for school and college students.
Essay on Animal Tissue Culture
Essay Contents:
- Essay on the Introduction to Animal Tissue Culture
- Essay on the Development and Maintenance of Cell Culture
- Essay on the Tissue Culture Facilities Required in Laboratory
- Essay on the Media Used for Animal Tissue Culture
- Essay on the Cell Culture and Its Types
- Essay on the Hybridoma Technology
- Essay on the Applications of Animal Cell Culture
1. Essay on the Introduction to Animal Tissue Culture:
Tissue Culture is the general term for the removal of cells, tissues, or organs from an animal or plant and their subsequent placement into an artificial environment favorable to growth. This environment usually consists of a suitable glass or plastic culture vessel containing a liquid or semisolid medium that supplies the nutrients essential for survival and growth. The culture of whole organs or intact organ fragments with the intent of studying their continued function or development is called organ culture. When the cells are removed from the organ fragments prior to, or during cultivation, thus disrupting their normal relationships with neighboring cells, it is called cell culture. Cell culture is the process by which cells are grown under controlled conditions. Cell culture has become one of the major tools used in the life sciences today.
In the beginning of 19th century, Sydney Ringer, English physiologist developed salt solutions containing the chlorides of sodium, potassium, calcium and magnesium suitable for maintaining the beating of an isolated animal heart outside of the body. In 1885, Wilhelm Roux removed a portion of the medullary plate of an embryonic chicken and maintained it in a warm saline solution for several days, establishing the principle of tissue culture. Rose Harrison (1907) developed a reproducible technique for tissue culture and later Alexis Carrel (1912) used tissue and embryo extracts as culture media.
They successfully demonstrated that animal cells can be grown indefinitely in vitro, just like protozoa and microorganisms. Although animal cell culture was first successfully undertaken by Ross Harrison in 1907, it was not until the late 1940s to early 1950s that several developments occurred that made cell culture widely available as a tool for scientists.
First, there was the development of antibiotics that made it easier to avoid many of the contamination problems that plagued earlier cell culture attempts. Second, was the development of the techniques, such as the use of trypsin to remove cells from culture vessels, necessary to obtain continuously growing cell lines (such as HeLa cells). Third, using these cell lines, scientists were able to develop standardized, chemically defined culture media that made it far easier to grow cells. These three areas combined to allow many more scientists to use cell, tissue and organ culture in their research.
2. Essay on the Development and Maintenance of Cell Culture:
Tissue culture is often a generic term that refers to both organ culture and cell culture and the terms are often used interchangeably. Cell cultures are derived from either primary tissue explants or cell suspensions. Primary cell cultures typically will have a finite life span in culture whereas continuous cell lines are, by definition, abnormal and are often transformed cell lines.
When cells are surgically removed from an organism and placed into a suitable culture environment, they will attach, divide and grow. This is called a primary culture. There are two basic methods for doing this. First, for explant cultures, small pieces of tissue are attached to a glass or treated plastic culture vessel containing suitable culture medium. After a few days, individual cells will move from the tissue explant out onto the culture vessel surface or substrate, where they will begin to divide and grow.
The second, more widely used method speeds up this process by adding digesting (proteolytic) enzymes, such as trypsin or collagenase, to the tissue fragments to dissolve the cement holding the cells together. This creates a suspension of single cells that are then placed into culture vessels containing culture medium and allowed to grow and divide. This method is called sub-culturing.
When the cells in the primary culture vessel have grown and filled up all of the available culture substrate, they must be sub-cultured to give them room for continued growth. This is usually done by removing them as gently as possible from the substrate with enzymes. These are similar to the enzymes used in obtaining the primary culture and are used to break the protein bonds attaching the cells to the substrate.
Once released, the cell suspension can then be subdivided and placed into new culture vessels. Once a surplus of cells is available, they can be treated with suitable cryoprotective agents, such as dimethylsulfoxide (DMSO) or glycerol, carefully frozen and then stored at cryogenic temperatures (below -130°C) until they are needed. Organizations such as American Type Culture Collection (ATCC) also provide high quality cell lines that are carefully tested to ensure the authenticity of the cells.
The basic culture systems can be of two types, based on the ability of the cells to either grow attached to a glass or treated plastic substrate (Monolayer culture systems) or floating free in the culture medium (suspension culture systems). Monolayer cultures are usually grown in tissue culture treated dishes, T-flasks, roller bottles, or multiple well plates, the choice being based on the number of cells needed, the nature of the culture environment, cost and personal preference.
Suspension cultures are usually grown either in magnetically rotated spinner flasks or shaken Erlenmeyer flasks where the cells are kept actively suspended in the medium. Many cell lines, especially those derived from normal tissues, are considered to be anchorage-dependent, that is, they can only grow when attached to a suitable substrate. Some cell lines that are no longer considered normal (transformed cells) are frequently able to grow either attached to a substrate or floating free in suspension; they are anchorage-independent. In addition, some normal cells, such as those found in the blood, do not normally attach to substrates and always grow in suspension.
Once in culture, cells exhibit a wide range of behaviors, characteristics and shapes. Cultured cells are usually described based on their morphology or their functional characteristics. The culture conditions play an important role in determining shape and that many cell cultures are capable of exhibiting multiple morphologies.
The three basic types of cells based on the morphologies are epithelial-like cells, which consists of cells that are attached to a substrate and appear flattened and polygonal in shape, the lymphoblast-like cells, here the cells that do not attach normally to a substrate but remain in suspension with a spherical shape and third type fibroblast like cells that are attached to a substrate and appear elongated and bipolar, frequently forming swirls in heavy cultures.
The characteristics of cultured cells result from both their origin (liver, heart, etc.) and how well they adapt to the culture conditions. Biochemical markers can be used to determine if cells are still carrying on specialized functions that they performed in vivo (e.g., liver cells secreting albumin). Some cell lines will eventually stop dividing and show signs of aging.
These lines are called finite. Other lines are, or become immortal; these can continue to divide indefinitely and are called continuous cell lines. When a “normal” finite cell line becomes immortal, it undergoes a fundamental irreversible change or “transformation”. This can occur spontaneously or be brought about intentionally using drugs, radiation or viruses. Transformed cells are usually easier and fast growing, may often have extra or abnormal chromosomes such as aneuploidy and frequently can be grown in suspension.
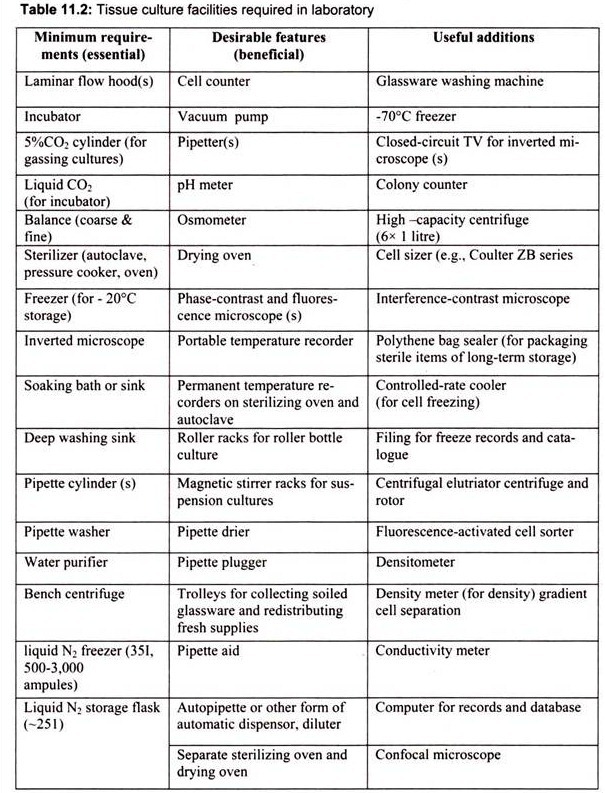
3. Essay on the Tissue Culture Facilities Required in Laboratory:
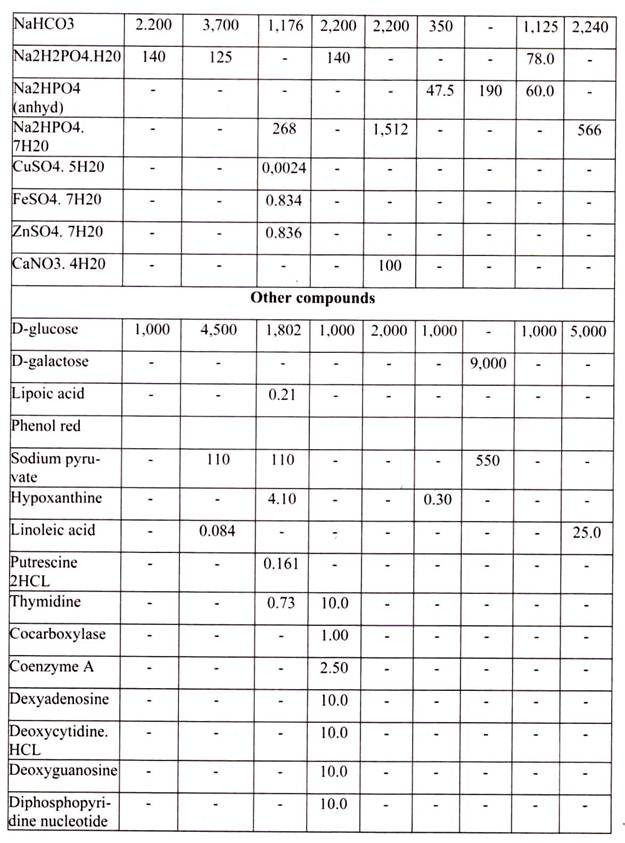
The laboratory facilities for animal culture should include facilities for sterile handling, incubation of cultured cells, glassware needed for preparation and handling of media and tissue, sterilization and storage. Tissue culture facilities required in any laboratory can be divided into essential and beneficial requirements on the basis of priority (Table 11.2).
Growth Media:
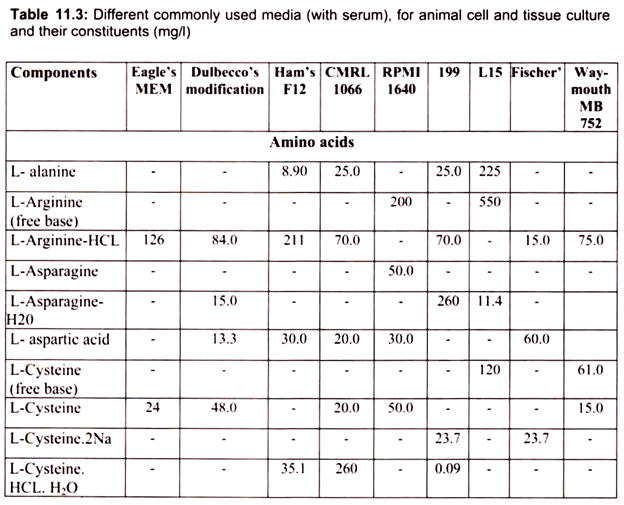
For growing cells in vitro, the environment should be as close as that expected in vitro. The nutrient media used for animal cell and tissue culture must be able to support their survival as well as growth, i.e. must provide nutritional, hormonal and stromal factors.
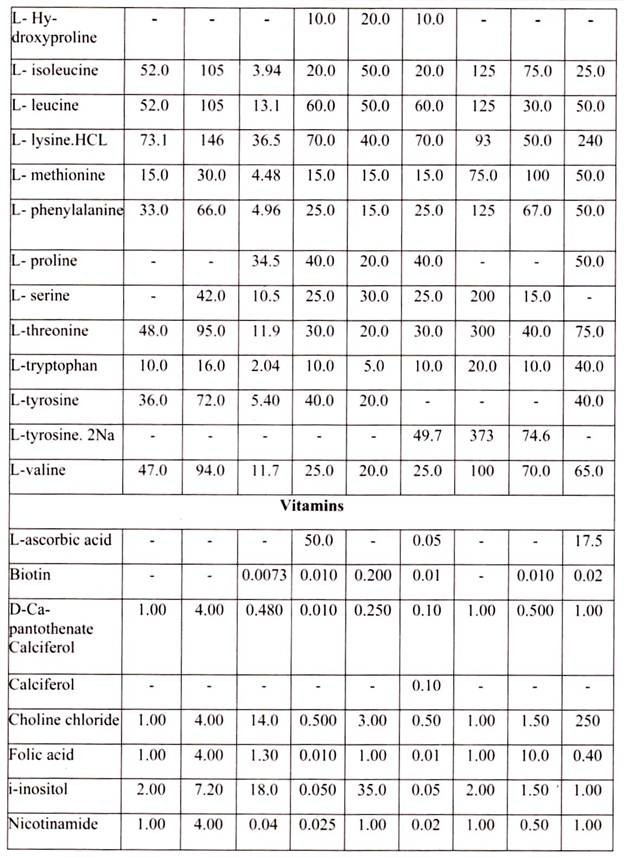
Nine amino acids, referred to as the essential amino acids, cannot be synthesized by adult vertebrate animals and thus must be obtained from their diet. Animal cells grown in culture also must be supplied with these nine amino acids, namely, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. In addition, most cultured cells require cysteine, glutamine and tyrosine.
In the intact animal, these three amino acids are synthesized by specialized cells; for example, liver cells make tyrosine from phenylalanine, and both liver and kidney cells can make glutamine. Animal cells both within the organism and in culture can synthesize the 8 remaining amino acids; thus these amino acids need not be present in the diet or culture medium.
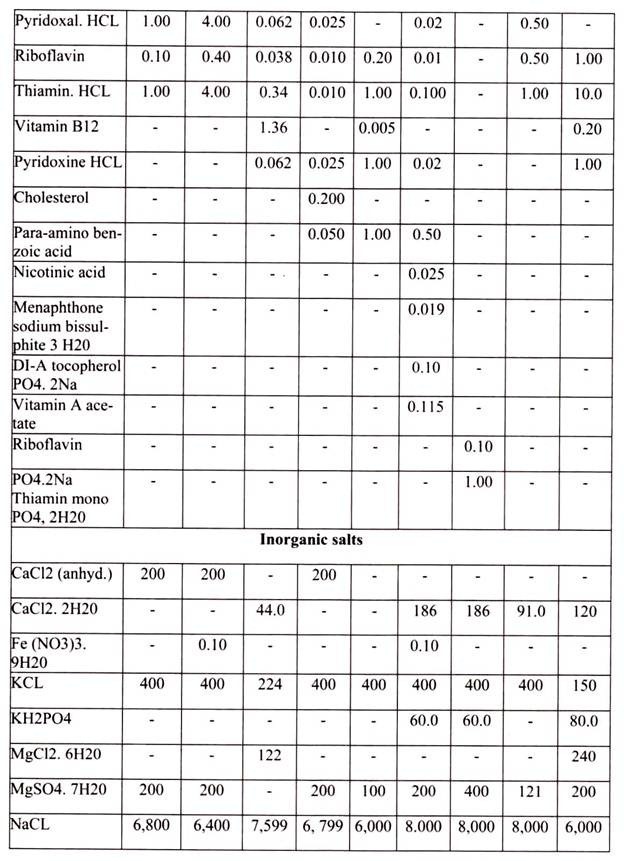
The other essential components of medium for culturing animal cells are Vitamins, which the cells cannot make at all or in adequate amounts; various salts, glucose and serum, the nuclear part of the blood. Serum, a mixture of hundreds of proteins, contains various factors needed for proliferation of cells in the culture.
For example, it contains insulin, a hormone required of many cultured vertebrate cells, and transferrin, an iron transporting protein essential for incorporation of iron by cells in culture. Although, many animal cells can grow in a serum-containing medium, such as Eagle’s medium, certain cell types require specific protein growth factors, which are not present in serum. For instance, precursors of red blood cells require the hormone erythropoietin, and T lymphocytes of the immune system require interleukin 2 (IL-2).
These factors bind to receptor proteins that span the plasma membrane, signaling the cells to increase in size and mass and undergo cell division. The medium is an important factor in this.
4. Essay on the Media Used for Animal Tissue Culture:
a. Natural Media:
Natural media is the most preferred medium, since it is the cheapest and most convenient.
The natural media, which promotes cell growth, falls into the following categories:
(a) Coagula such as Plasma Clots:
Plasma clots have been in use and plasma can be collected from male fowl, where efforts are made to prevent coagulation of blood by adding heparin as an anticoagulant.
(b) Biological Fluids such as Serum:
The most commonly used biological fluid is serum, which is obtained from human adult blood, placental cord blood, horse blood or calf blood. Out of these, human placental cord serum and foetal calf serum satisfactory. The serum can be obtained as exuded liquid from blood undergoing coagulation and is filtered using millipore filters. The serum should be tested for sterility and toxicity before using and stored at low temperature. The toxicity of sera can be reduced by heat inactivation. Besides serum, other biological fluids used as natural media includes amniotic fluid, ascitic fluid and pleural fluid, aqueous humour (from eye), serum ultrafiltrate and insect haemolymph.
b. Artificial Media:
The various artificial media developed for cell culture may be grouped into 4 classes:
(a) Serum-Containing media
(b) Serum-Free media
(c) Chemically defined media and
(d) Protein-Free media.
(a) Serum-Containing Media:
The various defined media, such as Eagle’s minimum essential media, when supplemented with 5-20% serum are good nutrient media for culture of most types of cells. The serum provides various plasma proteins, peptides, lipids, carbohydrates, minerals, and some enzymes. Serum serves several major functions like providing basic nutrients for cells, hormones, binding proteins and several minerals. It contains several growth factors and protease inhibitors. A major role of serum is to supply proteins and also act as a buffer. However, there are few disadvantages like it may inhibit growth of some cell types, like epidermal keratinocytes. Also the serum may contain cytotoxic constituents. Sometimes serum may interfere with downstream processing.
(b) Serum-Free Media:
Considering the disadvantages due to serum, extensive investigations have been made to develop serum-free formulations of culture media. These efforts were mainly based on analytical approach based on the analysis of serum constituents, synthetic approach to supplement basal media by various combinations of growth factors and the limiting-factor approach consisting of lowering the serum level in the medium till growth stops and then supplementing the medium with vitamins, amino acids, hormones, etc., till growth resumes.
Serum-free media has the advantages such as improved reproducibility of results over time from different laboratories since variation due to batch change of serum is avoided. It made easier downstream processing of products from cultured cells. Bioassays are free from interference due to serum proteins. There is no danger of degradation of sensitive proteins by serum proteases. They permit selective culture of differentiated and reproducing cell types from the heterogeneous cultures. It also has some disadvantages like most of them are specific to one cell type and the growth rate and the maximum cell density attained are lower than those with serum-containing media.
(c) Chemically Defined Media:
These media contain contamination-free ultra-pure inorganic and organic constituents, and may contain pure protein additives like insulin, epidermal growth factors, etc., that have been produced in bacteria or yeast by genetic engineering.
(d) Protein-Free Media:
In contrast to the above, protein-free media do not contain any protein; they only contain non-protein constituents necessary for culture of the cells.
In addition to the basic nutritional requirement of the cells, the medium should also control the pH range of the culture and buffers from abrupt changes. Usually a CO2– bicarbonate based buffer or an organic buffer, such as HEPES (4-(2-hydroxyethyl)-l-piperazine- ethanesulfonic acid) is a zwitterionic organic chemical buffering agent and is used to help, keep the medium pH in a range from 7.0 to 7.4 depending on the type of cell being cultured. When using a CO2-bicarbonate buffer, it is necessary to regulate the amount of CO2 dissolved in the medium. This is usually done using an incubator with CO2 controls set to provide an atmosphere with between 2% and 10% CO2 (for Eagle’s salts-based buffers).
However, some media use a CO2-bicarbonate buffer that requires no additional CO2, but it must be used in a sealed vessel (not dishes or plates). Finally, the osmolality (osmotic pressure) of the culture medium is important since it helps to regulate the flow of substances in and out of the cell. It is controlled by the addition or subtraction of salt in the culture medium. Evaporation of culture media from open culture vessels will rapidly increase the osmolality of the medium which affects the cell attachment. Appropriate medium and incubator that maintains the correct pH and osmolality is necessary to maintain proper growth rate.
The viability of cells can be observed visually using an inverted phase contrast microscope. Live cells are phase bright; suspension cells are typically rounded and somewhat symmetrical; adherent cells will form projections when they attach to the growth surface. Viability can also be assessed using the vital dye, trypan blue, which is excluded by live cells but accumulates in dead cells. Cell numbers are determined using a hemocytometer.
5. Essay on the Cell Culture and Its Types:
Cell culture is defined as the technique in which tissue or outgrowth from an explant is dispersed, most enzymatically, into a cell suspension, which may then be cultured as a monolayer or suspension culture. Some normal functions may be maintained but the original organization of tissue is lost.
Cell cultures may contain the following three types of cells:
1. Stem cells
2. Precursor cells
3. Differentiated cells
Stem cells are undifferentiated cells, which can differentiate under correct inducing conditions into one of sever kinds of cells. Different kinds of stem cells differ markedly in terms of the kinds of cells they will differentiate into. Precursor cells are derived from stem cells and are committed to differentiation. These cells retain the capacity for proliferation. In contrast, differentiated cells usually do not have the capacity to divide.
Some cell cultures like epidermal keratinocyte cultures contain all the three types of cells. In such cell cultures, stem cells constantly provide new cells, which develop into precursors; the precursor cells proliferate and mature into the differentiated cell types. Thus stem cells are necessary for the maintenance of such cultures, which by nature are heterogeneous. On the other hand fibroblast cultures contain a more or less uniform population of dividing cells at low cell densities ( <104 cells/cm2), but at high cell densities (105 cells/cm2) are uniformly composed of non- proliferating differentiated cells. The cells begin to proliferate once the cell density is approximately reduced.
Differentiation and cell proliferation are affected not only by cell density but also by factors like serum, Ca2+ ions, hormones, cell-to-cell and cell-to-matrix interactions, etc. Generally cell proliferation is promoted by low cell density, low Ca2+ (100-600 µm), and high growth factor levels, while differentiation is promoted by the exact opposite conditions and by the presence of differentiation-inducing factors, e.g. cortisone nerve growth factor, etc.
The proportion of stem, precursor and differentiated cells are markedly affected by the source tissue used for obtaining the cultures. For example, cultures derived from embryos and those derived from even adult tissues where continuous cell renewal occurs naturally (intestinal epithelium, haemopoietic cells, etc.), stem cells are likely to be more frequent than in other cell cultures. In contrast, cell cultures from tissues where renewal occurs only under stress, e.g. fibroblasts, muscle, etc., may contain only precursor cells.
Cell cultures can be grown as monolayers (adherent cell lines) or as suspension cultures (non-adherent cell lines). Propagation in suspension cultures is limited to haemopoietic cell lines, ascites, tumours and transformed cells (those cells that have become phenotypically modified during in vitro culture to become anchorage-independent and are able to grow in layers of several cells thick, as against monolayer growth of non-transformed cells). Therefore cells in culture need a surface or substrate to adhere to so that they are able to proliferate. Cells that are unable to adhere to a substrate are unable to divide, i.e., their growth is anchorage-dependent.
Cells of both adherent and non-adherent types may be:
(i) Primary cell lines,
( ii) Immortal ceil lines or
(iii) Transformed cell lines.
Primary cell cultures are established by inoculating growth medium with cells taken from animal tissue. The excised tissue is fragmented into small pieces with forceps and scissors. Then it is placed in sterile medium in a petri dish. The excised fragment is then treated with proteolytic enzymes such as trypsin to desegregate the tissue into individual cells. Such a culture may contain variety of differentiated cell types. In case of connective tissue, fibroblasts start growing within 7 days. Fibroblasts outgrow other cell types. Careful control of medium composition allows selective growth of certain cell types. Subculture of primary culture leads to secondary and tertiary cultures. Primary cell lines have limited lifespan and are also called finite cell cultures.
Cells from primary cultures multiply at a constant rate over successive transfers and such cells comprise a cell strain. Human cells generally divide only 50-100 times before dying. The natural aging of cells can be arrested by storage in liquid nitrogen. Immortal cell lines are capable of unlimited growth in culture and they do not die. They are altered to produce a continuous cell line from cells of primary culture. They are not necessarily malignant cells.
To be able to clone individual cells, modify cell behavior, or select mutants, biologists often want to maintain cell cultures for many more than 100 doublings. This is possible with cells derived from some tumors and with rare cells that arise spontaneously because they have undergone genetic changes that endow them with the ability to grow indefinitely. The genetic changes that allow these cells to grow indefinitely are collectively called oncogenic transformation, and the cells are said to be oncogenic ally transformed, or simply transformed. A culture of cells with an indefinite life span is considered immortal; such a culture is called a cell line to distinguish it from an impermanent cell strain.
The ability of cultured cells to grow indefinitely or their tendency to be transformed varies depending on the animal species from which the cells originate. Normal chicken cells rarely are transformed and die out after only a few doublings; even tumor cells from chickens almost never exhibit immortality. Among human cells, only tumor cells grow indefinitely.
The HeLa cell, the first human cell type to be grown in culture, was originally obtained in 1952 from a malignant tumor (carcinoma) of the uterine cervix. This cell line has been invaluable for research on human cells. During repeated serial transfer, cell lines can undergo extensive changes in their cultural properties; such cells may grow in clumps rather than in a monolayer, and cells may also be irregularly oriented with respect to each other.
Such cells are said to be transformed and are generally neoplastic. Transformed cell lines are either cells derived from tumour cells or cells manipulated by transfection with oncogenes, or obtained by treatment with carcinogens to produce cells with novel phenotype. These cell lines are quite robust. They have low doubling time and low requirement for growth factors. Although transformed cells are much easier to grow in culture, there is some reluctance to use them for large-scale production of biological. The concern is due to fear of contamination of products with tumorigenic agents.
6. Essay on the Hybridoma Technology:
Antibodies are proteins produced by the B lymphocytes of the immune system in response to foreign proteins, called antigens. Antibodies function as markers, binding to the antigen so that the antigen molecules can be recognized and destroyed by phagocytes. The part of the antigen that the antibody binds to is called the epitope. The epitope is thus a short amino acid sequence that enables the antibody to recognize. Two features of the antibody-epitope relationship of monoclonal antibodies such as specificity, the antibody binds only to its particular epitope and sufficiency, the epitope can bind to the antibody on its own, i.e. the presence of the whole antigen molecule is not necessary make it as a molecular tool.
In 1975 Cesar Milstein, Georges Kohler and Niels Jeme developed monoclonal antibody technology by fusing immortal tumor cells (myeloma cells) with antibody-producing B lymphocyte cells to produce “hybridomas,” that continuously synthesize identical (or “monoclonal”) antibodies. Milstein, Kohler and Jeme were awarded the 1984 Nobel Prize in Medicine.
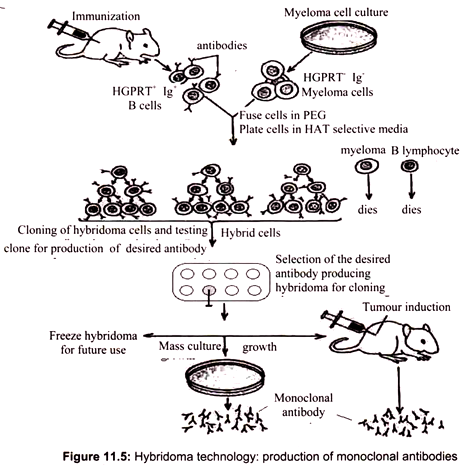
For isolation of B lymphocyte producing a certain antibody, we first have to induce the production of such a B cell in an organism. This is typically done in two doses, an initial “priming” dose and a second “booster” dose, 10 days later. Since the protein is of foreign origin, the mouse immune system recognizes it as such and soon some of the B cells in the mouse would begin production of the antibody against antigen. A sample of B cells is extracted from the spleen of the mouse and added to a culture of myeloma cells (cancer cells). The intended result is the formation of hybridomas, cells formed by the fusion of a B cell and a myeloma cell. The fusion is done by using polyethylene glycol, a virus or by electroporation.
The next step is to select for the hybridomas. The myeloma cells are HGPRT- and the B cells are HGPRT+. HGPRT is hypoxanthine-guanine phosphoribosyl transferase, an enzyme involved in the synthesis of nucleotides from hypoxanthine, an amino acid. The culture is grown in HAT (hypoxanthine-aminopterin-thymine) medium, which can sustain only HGPRT+ cells. The myeloma cells that fuse with another myeloma cell or do not fuse at all die in the HAT medium since they are HGPRT-. The B cells that fuse with another B cell or do not fuse at all die because they do not have the capacity to divide indefinitely. Only hybridomas between B cells and myeloma cells survive, being both HGPRT+ and cancerous.
The initial collection of B cells used is heterogenous, i.e. they do not all produce the same antibody. Therefore the hybridoma population too does not produce a single antibody. There is also another complication. A hybridoma cell is initially tetraploid, having been formed by the fusion of two diploid cells. However the extra chromosomes are somehow lost in subsequent divisions in a random manner. This means that we cannot be certain that the hybridomas will all produce the desired antibody or even any antibody at all. Screening is required to decide which hybridoma cells are actually producing the desired antibody (Fig. 11.5).
Each hybridoma is cultured and screened after doing SDS-PAGE (sodium dodecyl sulfate – polyacrylamide gel electrophoresis) and Western blots. The probe used is the epitope of the antibody that is desired, which may be labeled by radioactivity or immunofluorescence. Once it is sure that a certain hybridoma is producing the right antibody, it can be indefinitely cultured and monoclonal antibodies can be harvested from it. The resulting hybridomas can produce large quantities of the desired antibody. These antibodies, called monoclonal antibodies due to their purity, have many important clinical, diagnostic, and industrial applications with a yearly value of well over a billion dollars.
7. Essay on the Applications of Animal Cell Culture:
Cell culture has become one of the major tools used in cell and molecular biology. Some of the important areas where cell culture is currently playing a major role for studying basic cell biology and biochemistry, the interactions between disease-causing agents and cells, the effects of drugs on cells, the process and triggers for aging, and nutritional studies. A variety of areas of research in biotechnology depend largely on tissue culture and, therefore, these areas have given the desired impetus to the development of tissue culture methods in animals.
These areas include the following:
(i) Production of antiviral vaccines, which required the standardization of cell lines for the multiplication and assay of viruses;
(ii) Cancer research, which required the study of uncontrolled cell division in cultures;
(iii) Cell fusion techniques;
(iv) Genetic manipulation, which is possible in cells or organs in culture;
(v) Monoclonal antibodies whose production, requires cell lines in culture;
(vi) Production of pharmaceutical drugs using cell lines;
(vii) Chromosome analysis of cells derived from womb;
(viii) Study of the effects of toxins and pollutants using cell lines;
(ix) Use of artificial skin;
(x) Study of the function of nerve cells (even though neurons cannot be propagated in vitro, without resorting to the use of transformed cells).
The major research fields in which cell lines find applications include:
a. Toxicity Testing:
Cultured cells are widely used alone or in conjunction with animal tests to study the effects of new drugs, cosmetics and chemicals on survival and growth in a wide variety of cell types. Especially important are liver- and kidney-derived cell cultures.
b. Cancer Research:
Since both normal cells and cancer cells can be grown in culture, the basic differences between them can be closely studied. In addition, it is possible, by the use of chemicals, viruses and radiation, to convert normal cultured cells to cancer causing cells. Thus, the mechanisms that cause the change can be studied.
c. Virology:
One of the earliest and major uses of cell culture is the replication of viruses in cell cultures (in place of animals) for use in vaccine production. Cell cultures are also widely used in the clinical detection and isolation of viruses, as well as basic research into how they grow and infect organisms.
d. Cell-Based Manufacturing:
While cultured cells can be used to produce many important products, three areas are generating the most interest. The first is the large-scale production of viruses for use in vaccine production. These include vaccines for polio, rabies, chicken pox, hepatitis B and measles, the large-scale production of cells that have been genetically engineered to produce proteins that have medicinal or commercial value.
These include monoclonal antibodies, insulin, hormones, etc. and the use of cells as replacement tissues and organs. Artificial skin for use in treating burns and ulcers is the first commercially available product. A potential supply of replacement cells and tissues may come out of work currently being done with both embryonic and adult stem cells. These are cells that have the potential to differentiate into a variety of different cell types and it may offer new treatment approaches for a wide variety of medical conditions.
e. Genetic Counseling:
Amniocentesis, a diagnostic technique that enables doctors to remove and culture fetal cells from pregnant women, an important tool for the early diagnosis of fetal disorders. These cells can then be examined for abnormalities in their chromosomes and genes using karyotyping, chromosome painting and other molecular techniques.
f. Genetic Engineering:
The ability to transfect or reprogram cultured cells with new genetic material (DNA and genes) has provided a major tool to molecular biologists wishing to study the cellular effects of the expression of these genes (new proteins). These techniques can also be used to produce these new proteins in large quantity in cultured cells for further study.
g. Gene Therapy:
The ability to genetically engineer cells has also led to their use for gene therapy. Cells can be removed from a patient lacking a functional gene and the missing or damaged gene can then be replaced. The cells can be grown for a while in culture and then replaced into the patient. An alternative approach is to place the missing gene into a viral vector and then “infect” the patient with the virus in the hope that the missing gene will then be expressed in the patient’s cells.
h. Drug Screening and Development:
Cell-based assays have become increasingly important for the pharmaceutical industry, not just for cytotoxicity testing but also for high throughput screening of compounds that may have potential use as drugs.