Are you looking for an essay on the ‘Immune System’? Find paragraphs, long and short essays on the ‘Immune System’ especially written for school and college students.
Biology Essay on Immune System
Essay Contents:
- Essay on the Introduction to Immune System
- Essay on the Organs of the Immune System
- Essay on the Fluid Systems of the Body
- Essay on the Cells of the Immune System
- Essay on the Immunoglobulin Classes
- Essay on the Cytokines: Major Sources and Significance
- Essay on the Complement System
- Essay on the Major Histocompatibility Complex
- Essay on the T Cell Receptor (TCR)
- Essay on the B- Cell Receptor
- Essay on the Antigens, Mitogens and Immunomodulating Substances
- Essay on the Vaccines
1. Essay on the Introduction to Immune System:
The immune system is a highly sensitive, responsive and adaptive versatile defence system that has evolved to protect animals from invading pathogenic microorganisms and cancer. It is able to generate an enormous variety of cells and molecules capable of specifically recognizing and eliminating an apparently limitless variety of foreign invaders. It protects us against billions of bacteria, viruses, and other parasites. Functionally, an immune response can be divided into two related activities—recognition and response. Immune recognition is remarkable for its specificity. It is able to recognize subtle chemical differences that distinguish one foreign pathogen from another and even discriminate between foreign molecules and the body’s own cells and proteins. Once a foreign organism has been recognized, the immune system recruits a variety of cells and molecules to mount an appropriate response, called as an effector response, that eliminates or neutralizes the foreign organism. Thus system becomes able to convert the initial recognition event into a variety of effector responses, each uniquely suited for eliminating a particular type of pathogen. Subsequent exposure to the same foreign organism induces a memory response, characterized by a more rapid and heightened immune reaction that serves to eliminate the pathogen and prevents the disease.
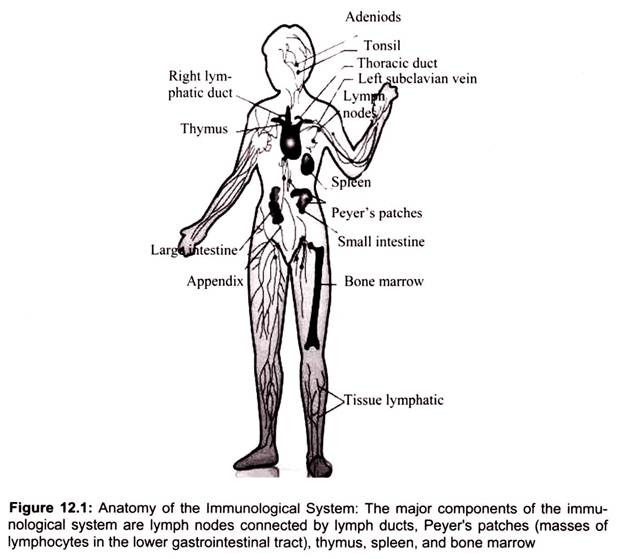
The immune system is composed of many interdependent cell types that interplay to protect the body from bacterial, parasitic, fungal, viral infections and from the growth of tumor cells (Fig. 12.1). Many of these cell types have specialized functions. The cells of the immune system can engulf bacteria, kill parasites or tumor cells, or kill viral-infected cells. Often, these cells depend on the T helper subset for activation signals in the form of secretions formally known as cytokines, lymphokines, or more specifically interleukins.
2. Essay on the Organs of the Immune System:
The thymus and bone marrow are the primary (or central) lymphoid organs, where maturation of lymphocytes takes place. The lymph nodes, spleen, and various mucosal associated lymphoid tissues (MALT) such as gut-associated lymphoid tissue (GALT) are the secondary (or peripheral) lymphoid organs, which trap antigen and provide sites for mature lymphocytes to interact with that antigen. In addition, tertiary lymphoid tissues, which normally contain fewer lymphoid cells than secondary lymphoid organs, can import lymphoid cells during an inflammatory response (Fig. 12.1).
a. Bone Marrow:
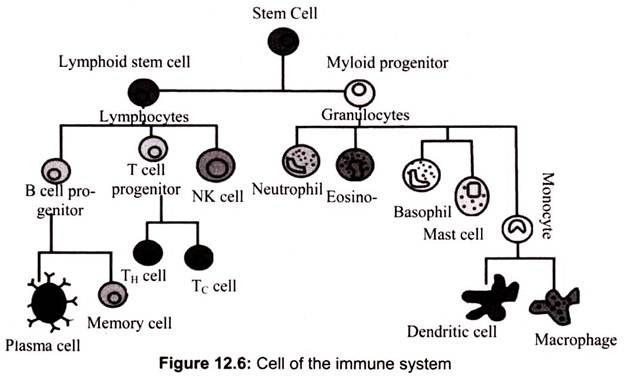
The cells of the immune system are basically derived from the bone marrow through a process called hematopoiesis. During this process, the bone marrow-derived stem cells differentiate into either mature cells of the immune system or into precursor cells that migrate out of the bone marrow to continue their maturation elsewhere like thymus, spleen or lymphatic tissues. The bone marrow produces B cells, natural killer cells, granulocytes and immature thymocytes, in addition to red blood cells and platelets.
b. Thymus:
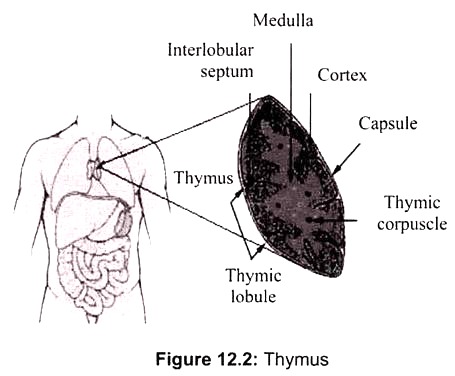
Thymus is the site of maturation of T cells. Immature thymocytes (prothymocytes) leave the bone marrow and migrate into the thymus, there they undergo remarkable maturation process, referred to as thymic education and are then released into the bloodstream. It is a flat, bilobed organ situated above the heart. Each lobe is surrounded by a capsule and is divided into lobules, which are separated from each other by strands of connective tissue called trabecule.
Each lobule is organized into two compartments- the outer compartment, or cortex, is densely packed with immature T cells, called thymocytes, whereas the inner compartment, or medulla, is sparsely populated with thymocytes (Fig. 12.2). The function of the thymus is to generate and select a repertoire of T cells that will protect the body from infection. As thymocytes develop, an enormous diversity of T-cell receptors is generated by a random process that produces some T cells with receptors capable of recognizing antigen-MHC complexes.
c. Spleen:
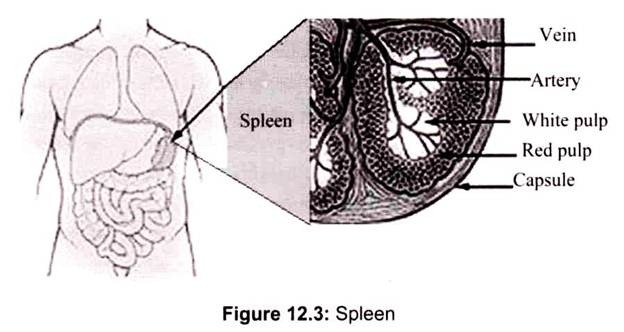
The spleen is an immunologic filter of the blood. It is a reserve of B cells, T cells, macrophages, dendritic cells, natural killer cells and red blood cells. It is a large, ovoid secondary lymphoid organ situated high in the left abdominal cavity. While lymph nodes are specialized for trapping antigen from local tissues, the spleen specializes in filtering blood and trapping blood-borne antigens; thus, it can respond to systemic infections. Unlike the lymph nodes, the spleen is not supplied by lymphatic vessels. Instead, blood borne antigens and lymphocytes are carried into the spleen through the splenic artery.
In addition to capturing foreign materials (antigens) from the blood that passes through the spleen, migratory macrophages and dendritic cells bring antigens to the spleen via the bloodstream. An immune response is initiated when the macrophage or dendritic cells present the antigen to the appropriate B or T cells. In the spleen, B cells become activated and produce large amounts of antibody. Also, old red blood cells are destroyed in the spleen (Fig. 12.3).
d. Lymph Nodes:
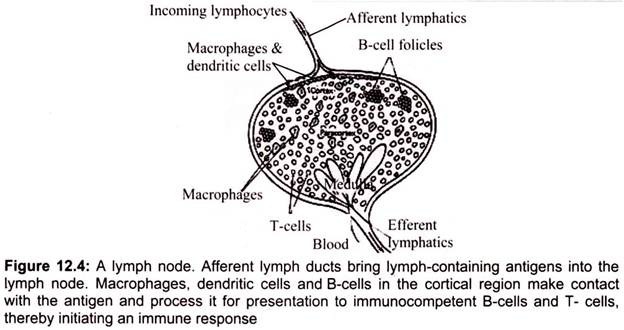
They are encapsulated bean shaped structures containing a reticular network packed with lymphocytes, macrophages, and dendritic cells. Lymph nodes are found throughout the body and serves as an immunologic filter for the bodily fluid known as lymph. They are clustered at junctions of the lymphatic vessels; lymph nodes are the first organized lymphoid structures which encounter antigens that enter the tissue spaces (Fig. 12.4). As lymph percolates through a node, any particulate antigen that is brought in with the lymph gets trapped by the cellular network of phagocytic cells and dendritic cells.
Antigens are filtered out of the lymph in the lymph node returning the lymph is returned to the circulation in a similar fashion as the spleen, the macrophages and dendritic cells that capture antigens present these foreign materials to T and B cells, consequently initiating an immune response. As antigen is carried to a regional node by the lymph, it is trapped, processed, and presented together with class II MHC molecules by interdigitating dendritic cells in the paracortex, resulting in the activation of TH cells.
3. Essay on the Fluid Systems of the Body:
There are two main dynamic fluid systems exist in the body namely blood and lymph. The blood and lymph systems are intertwined throughout the body and they are mainly responsible for transporting the agents of the immune system.
a. The Blood System:
Blood is the major transporter of the body, which flows from the heart into arteries, then to capillaries, and returns to the heart through veins. On an average the body of 70 kg person contains 5 liters of blood, which constitutes about 7% of the total body weight. Blood is composed of 52-62% liquid plasma and 38-48% cells. The plasma is mostly water (91.5%) and acts as a vehicle for transporting other materials (7% protein [consisting of albumins (54%), globulins (38%), fibrinogen (7%), and assorted other remaining stuffs]). Blood is slightly alkaline (pH = 7.40 ± .05) and heavier than water (density = 1.057 ± .009).
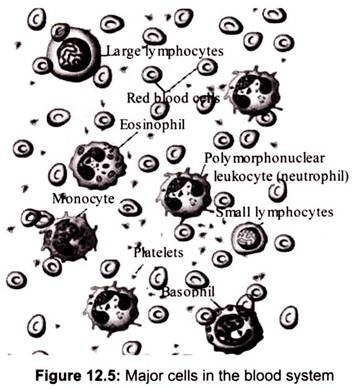
Blood cells are derived from hematopoietic stem cells, which are mainly found in the bone marrow, via a process called hematopoiesis. The stem cells produce hemocytoblasts that differentiate into the precursors for all the different types of blood cells (Fig. 12.5). Hemocytoblasts mature into three types of blood cells: erythrocytes (red blood cells or RBCs), leukocytes (white blood cells) and platelets.
The leukocytes are further subdivided into granulocytes (containing large granules in the cytoplasm) and agranulocytes (without granules). The granulocytes are consisted of neutrophils (55-70%), eosinophils (1-3%), and basophils (0.5-1.0%). The agranulocytes are lymphocytes (consisting of B cells and T cells) and monocytes. Lymphocytes circulate in the blood and lymph systems, and make their home in the lymphoid organs.
There are 5000-10,000 WBCs per mm3 and biological life is 5-9 days. About 2,400,000 RBCs are produced each second and each of them of them lives for about 120 days (They migrate to the spleen to die. Once there, that organ scavenges usable proteins from their carcasses). A healthy male has about 5 million RBCs per mm3, whereas females have a bit fewer than 5 million.
b. The Lymph System:
Lymph is an alkaline (pH > 7.0) fluid that is usually clear, transparent, and colorless. It flows in the lymphatic vessels and bathes tissues and organs in its protective covering. There are no RBCs in lymph and it has relatively lower protein content than blood. Like blood, it is slightly heavier than water (density = 1.019 ± .003).
The lymph flows from the interstitial fluid through lymphatic vessels up to either the thoracic duct or right lymph duct, which terminates in the subclavian veins, where lymph is mixed with the blood. (The right lymph duct drains the right sides of the thorax, neck, and head, whereas the thoracic duct drains the rest of the body.) Lymph carries lipids and lipid- soluble vitamins absorbed from the gastrointestinal (GI) tract. Since there is no active pump in the lymph system, there is no back-pressure produced. The lymphatic vessels, like veins, have one-way valves that prevent backflow. Additionally, along these vessels there are small bean-shaped lymph nodes which serve as filters of the lymphatic fluid. It is in the lymph nodes where antigen is usually presented to the immune system.
The human lymphoid system consists of the followings:
i. Primary organs- bone marrow (in the hollow center of bones) and the thymus gland (located behind the breastbone above the heart), and
ii. Secondary organs at or near possible portals of entry for pathogens: adenoids, tonsils, spleen (located at the upper left of the abdomen), lymph nodes (along the lymphatic vessels with concentrations in the neck, armpits, abdomen, and groin), Peyer’s patches (within the intestines), and the appendix.
Lymphocytes originate in the bone marrow but migrate to the parts of the lymphatic system such as the lymph nodes, spleen, and thymus. There are two main types of lymphatic cells, T cells and B cells. The lymphatic system also involves a transportation system – lymph vessels – for transportation and storage of lymphocyte cells within the body. The lymphatic system feeds cells into the body while filters out dead cells and invading organisms such as bacteria.
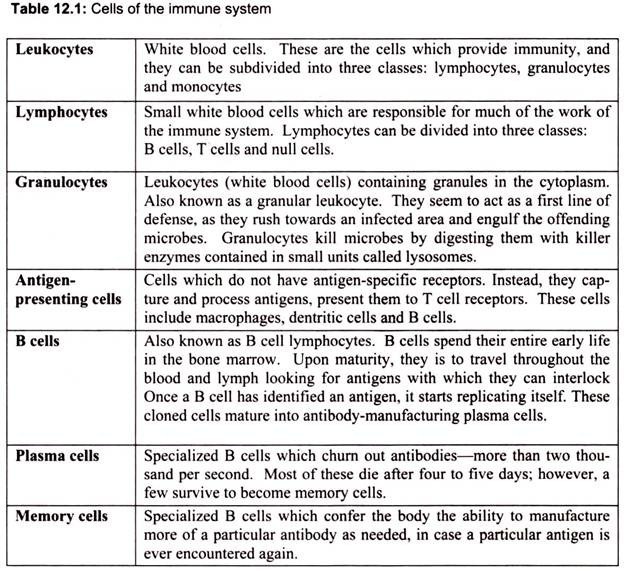
4. Essay on the Cells of the Immune System:
T cell-dependent acquired immune responses typically require antigen-presenting cells (APCs) to present Ag-derived peptides within major histocompatibility complex (MHC) molecules though some antigens can stimulate the immune response directly. Intracellular Ag (e.g., viruses) can be processed and presented to CD8+ cytotoxic T cells by any nucleated cell because all nucleated cells express class I MHC molecules. However, extracellular antigen must be processed into peptides and complexed with surface class II MHC molecules present on professional APCs to be recognized by CD4+ helper T (TH) cells.
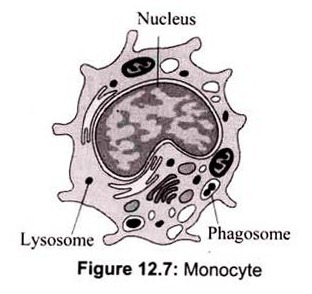
a. Monocytes:
Monocytes in the circulation are precursors to tissue macrophages. During hematopoiesis in the bone marrow, granulocyte-monocyte progenitor cells differentiate into promonocytes, which leave the bone marrow and enter the blood, where they further differentiate into mature monocytes. Monocytes migrate into tissues, where over about 8 h, they develop into macrophages under the influence of macrophage colony-stimulating factor (M- CSF), secreted by various cell types (e.g., endothelial cells, fibroblasts). At infection sites, activated T cells secrete cytokines (e.g., interferon-γ [IFN-γ]) that induce production of macrophage migration inhibitory factor, preventing macrophages migration (Fig. 12.7).
T cells got the name from its organ of maturation thymus, an organ situated under the breast bone. T cells are produced in the bone marrow and later move to the thymus where they mature. T lymphocytes are usually divided into two major subsets that are functionally and phenotypically different. The T helper subset, also called the CD4+ T cell, is a pertinent coordinator of immune regulation. The main function of the T helper cell is to augment or potentiate immune responses by the secretion of specialized factors that activate other white blood cells to fight off infection.
Another important type of T cell is called the T killer/suppressor subset or CD8+ T cell. These cells are important in directly killing certain tumor cells, viral-infected cells and sometimes parasites. The CD8+ T cells are also important in down-regulation of immune responses. Both types of T cells can be found throughout the body. They often depend on the secondary lymphoid organs (the lymph nodes and spleen) as sites where activation occurs, but they are also found in other tissues of the body, most conspicuously the liver, lung, blood, and intestinal and reproductive tracts.
i. Helper T Cells:
Helper T cells are the major driving force and the main regulators of the immune defense. Their primary task is to activate B cells and killer T cells. However, the helper T cells themselves must be activated. This happens when a macrophage or dendritic cell, which has uptaken an invader, travels to the nearest lymph node to present information about the captured pathogen. The phagocyte displays an antigen fragment from the invader on its own surface, a process called antigen presentation. When the receptor of a helper T cell recognizes the antigen, the T cell is activated. Once activated, helper T cells start to divide and to produce proteins that activate B and T cells as well as other immune cells.
ii. The Killer T Cell:
The killer T cell is specialized in attacking cells of the body infected by viruses and sometimes also by bacteria. It can also attack cancer cells. The killer T cell has receptors that are used to search each cell that it meets. If a cell is infected, it is swiftly killed. Infected cells are recognized because tiny traces of the intruder, antigen, can be found on their surface.
iii. Natural Killer Cells:
Natural killer cells, often referred to as NK cells, are similar to the killer T cell subset (CD8+ T cells). They function as effector cells that directly kill certain tumors such as melanomas, lymphomas and viral-infected cells, most notably herpes and cytomegalovirus- infected cells. NK cells, unlike the CD8+ (killer) T cells, kill their targets without a prior “conference” in the lymphoid organs. However, NK cells that have been activated by secretions from CD4+ T cells will kill their tumor or viral-infected targets more effectively.
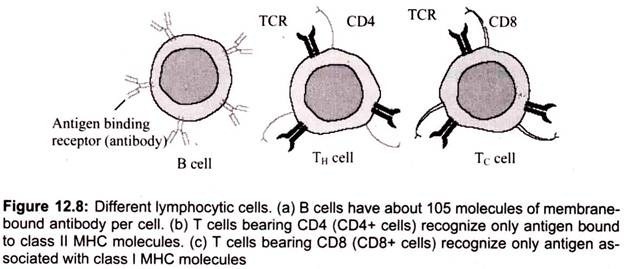
c. B Cells:
The major function of B lymphocytes is the production of antibodies in response to foreign proteins of bacteria, viruses, and tumor cells. Antibodies are specialized proteins that specifically recognize and bind to antigen. Antibody production and binding to a foreign substance or antigen, often is critical as a means of signaling other cells to engulf, kill or remove that substance from the body. The B lymphocyte cell searches for antigen matching its receptors. If it finds such antigen it connects to it, and inside the B cell a triggering signal is set off. The B cell now needs proteins produced by helper T cells to become fully activated. When this happens, the B cell starts to divide to produce clones of it. During this process, two new cell types are created, plasma cells and B memory cells.
i. The Plasma Cell:
The plasma cell is specialized in producing a specific protein, called an antibody that will respond to the same antigen that matched the B cell receptor. Antibodies are released from the plasma cell so that they can seek out intruders and help destroy them. Plasma cells produce antibodies at an amazing rate and can release tens of thousands of antibodies per second. When the Y-shaped antibody finds a matching antigen, it attaches to it.
The attached antibodies serve as an appetizing coating for eater cells such as the macrophage. Antibodies also neutralize toxins and incapacitate viruses, preventing them from infecting new cells. Each branch of the Y-shaped antibody can bind to a different antigen, so while one branch binds to an antigen on one cell, the other branch could bind to another cell – in this way pathogens are gathered into larger groups that are easier for phagocyte cells to devour. Bacteria and other pathogens covered with antibodies are also more likely to be attacked by the proteins from the complement system.
ii. The Memory Cells:
The Memory Cells are the second cell type produced by the division of B cells. These cells have a prolonged life span and can thereby “remember” specific intruders. T cells can also produce memory cells with an even longer life span than B memory cells. The second time an intruder tries to invade the body, B and T memory cells help the immune system to activate much faster. The invaders are wiped out before the infected human feels any symptoms. The body has achieved immunity against the invader.
d. Granulocytes or Polymorphonuclear (PMN) Leukocytes:
Another group of white blood cells is collectively referred to as granulocytes or polymorphonuclear leukocytes (PMNs). Granulocytes are composed of three cell types identified as neutrophils, eosinophils and basophils, based on their staining characteristics with certain dyes. These cells are predominantly important in the removal of bacteria and parasites from the body. They engulf these foreign bodies and degrade them using their powerful enzymes.
e. Macrophages:
Macrophages are important in the regulation of immune responses. They are often referred to as scavengers or antigen-presenting cells (APC) because they pick up and ingest foreign materials and present these antigens to other cells of the immune system such as T cells and B cells. This is one of the important first steps in the initiation of an immune response. Stimulated macrophages exhibit increased levels of phagocytosis and are also secretory.
f. Dendritic Cells:
Another cell type, addressed only recently, is the dendritic cell. Dendritic cells, which also originate in the bone marrow, function as antigen presenting cells (APC). In fact, the dendritic cells are more efficient APCs than macrophages. These cells are usually found in the structural compartment of the lymphoid organs such as the thymus, lymph nodes and spleen. However, they are also found in the bloodstream and other tissues of the body. It is believed that they capture antigen or bring it to the lymphoid organs where an immune response is initiated.
g. Mast Cells:
Mast Cells are found in different tissues of the body. Mucosal mast cell granules contain tryptase and chondroitin sulfate; connective tissue mast cell granules contain tryptase, chymase, and heparin. By releasing these mediators, mast cells play a key role in generating protective acute inflammatory responses; basophils and mast cells are the source of type I hypersensitivity reactions associated with atopic allergy. Degranulation can be triggered by cross-linking of IgE receptors 6r by the anaphylatoxin complement fragments C3a and C5a.
h. Antigen Presenting Cells (APCs):
Induction of the humoral immune response begins with the recognition of antigen. Through a process of clonal selection, specific B-cells are stimulated to proliferate and differentiate. However, this process requires the intervention of specific T-cells that are themselves stimulated to produce lymphokines that are responsible for activation of the antigen-induced B- cells. In other words, B cells recognize antigen via immunoglobulin receptors on their surface but are unable to proliferate and differentiate unless prompted by the action of T-cell lymphokines. In order for the T-cells to become stimulated to release lymphokines, they must also recognize specific antigen. However, while T-cells recognize antigen via their T- cell receptors, they can only do so in the context of the MHC molecules. This “antigen- presentation” is the responsibility of the antigen-presenting cells (APCs).
Several types of cells may serve the APC function. Perhaps the best APC is, in fact, the B-cell itself. When B-cells bind antigen, the antigen becomes internalized, processed and expressed on the surface of the B-cell. Expression occurs within the class II MHC molecule, which can then be recognized by T-helper cells (CD4+).
Other types of antigen-presenting cells include the macrophage and dendritic cells. These cells either actively phagocytose or pinocytose foreign antigens. The antigens are then processed in a manner similar to that observed for the B-cells. Next, specific antigen epitopes are expressed on the macrophage or dendritic cell surface. Again, this expression occurs within the class II MHC molecule, where T-cell recognition occurs. The stimulated T-cells then release lymphokines that act upon “primed” B-cells (B-cells that have already encountered antigen), inducing B-cell proliferation and differentiation.
5. Essay on the Immunoglobulin Classes:
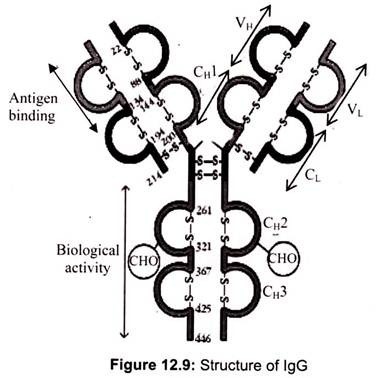
a. Immunoglobulin G (IgG):
70-75% of the total human serum immunoglobulin consists of Immunoglobulin G. IgG is a monomelic protein with two y heavy chains and two k or 1 light chains. It has a molecular weight of 1,46,000 with a sedimentation coefficient of 7S. The IgG class is distributed evenly between the intra and extra-vascular pools. It is the major antibody of secondary immune responses and the exclusive-toxin class. There are four IgG subclasses namely IgG 1, IgG2, IgG3 and IgG4 numbered in accordance with their order of occurrence in the serum. One important thing to be noted is that no two subclasses are identical.
This may be either with reference to the number or the distribution of inter-chain disulfide linkages. The four subclasses are encoded by different germ-line CH genes whose DNA sequences are 90-95% homologous. The differences in amino acid sequence in IgG subclasses are directly related to biological activity. While IgGl, IgG3 and IgG4 cross the placenta and play an important role in protecting the fetus, IgG2 crosses only partially in some cases.
Complement activation efficiency also differs; IgG3 is the most effective, followed by IgG 1, IgG2 and IgG4. The opsonin activity of IgG is attributed to its binding to Fc receptors on phagocytic cells. Again, the different subclasses have different affinity. IgGl and IgG3 have a higher affinity to Fc receptors while IgG4 has an intermediate affinity, IgG2 shows an extremely low affinity.
b. Immunoglobulin A (IgA):
Immunoglobulin A (IgA), the predominant immunoglobulin in seromucous secretions, constitutes 15-20% of the normal human serum immunoglobulin pool. It is protected from proteolysis by combination with another protein; the secretory component IgA is predominantly seen in external secretions such as breast milk, saliva, tears and mucus of the bronchial, genitourinary and digestive tracts. Hence, they are frequently known as secretory IgA (slgA).
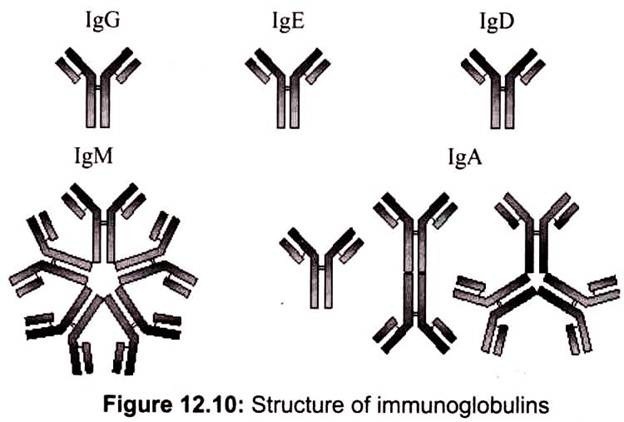
In serum, IgA exists as a monomer; however, in most cases it is polymeric, i.e. dimers, trimmers, etc. The slgA consists of a dimer or tetramer, a J chain polypeptide and a polypeptide chain called secretory component. The secretory component is a polypeptide with a molecular weight of 70,000 and is produced by epithelial cells of mucous membranes. It has five immunoglobulin like domains that bind to the Fc regions of the IgA dimmer (Fig. 12.10).
c. Immunoglobulin M (IgM):
IgM accounts for about 10% of the total serum immunoglobulin with an average serum concentration of 1.5% mg/ml. Unlike IgG or IgA, IgM has a pentameric structure in which individual heavy chains have a molecular weight of approximately 65,000 while the total molecular weight amounts to 9,70,000. The monomelic units consist of two m heavy chains and two light chains. The subunits are held together by disulfide bonds between their car- boxy 1 terminal (Cm 4/Cm4) domains and Cm3/Cm3.
The subunits are so arranged that their Fc region lies in the center of the pentamer. It is believed that IgM has 10 antigen binding sites but they are unable to combine with the antigen with similar efficiency; this makes it difficult to demonstrate the presence of 10 sites. In addition to the above, a Fc linked polypeptide called J (joining) chain, is disulfide bonded to the carboxyl terminal cysteine residue of two of the 10m chains.
The J chain appears to be required for polymerization of the monomer. The presence of the J chain allows IgM to bind to receptors on secretory cells, which helps them in transporting across the epithelial linings to external secretions that bathe mucosal surfaces. IgM is largely confined to the intravascular pool and is the predominant ‘early’ antibody frequently directed against antigenic complexes after a primary response. It is also the first to be synthesized by the neonate.
Monomeric IgM is expressed as membrane bound antibody on B cells. Because of its pentameric structure, serum IgM has higher valency than others. For example, it takes 100-1000 times as many molecules of IgG as of IgM to achieve the same level of agglutination. Due to its large size, diffusion to the intracellular tissue fluids is very low (fig. 12.10).
d. Immunoglobulin D (lgD):
IgD consfitutes less than 1 % of the total plasma immunoglobulin with a serum concentration of 30mg/ml. IgD, together with IgM, is the major membrane bound immunoglobulin expressed by mature B cells. Though their exact biological function is not clear they are thought to function in the activation of B cells by an antigen. IgD is more susceptible to proteolysis than any other immunoglobulin class. IgD has a simple disulfide bond between the d chains and a high content of carbohydrate distributed in multiple oligosaccharide units. One of these units is rich in N-acetyl galactosamine (Fig. 12.10).
e . Immunoglobulin E (IgE):
IgE is present in low levels in serum and in respiratory and gastro-intestinal mucous secretions. IgE binds with high affinity to receptors present in high levels on mast cells and basophils and to a lesser extent on several other hematopoietic cells, including dendritic cells. If Ag bridges 2 IgE molecules bound to the mast cell or basophil surface, the cells degranulate, releasing chemical mediators that cause an inflammatory response. IgE levels are elevated in atopic disorders (e.g., allergic or extrinsic asthma, hay fever, atopic dermatitis) and parasitic infections (Fig. 12.10).
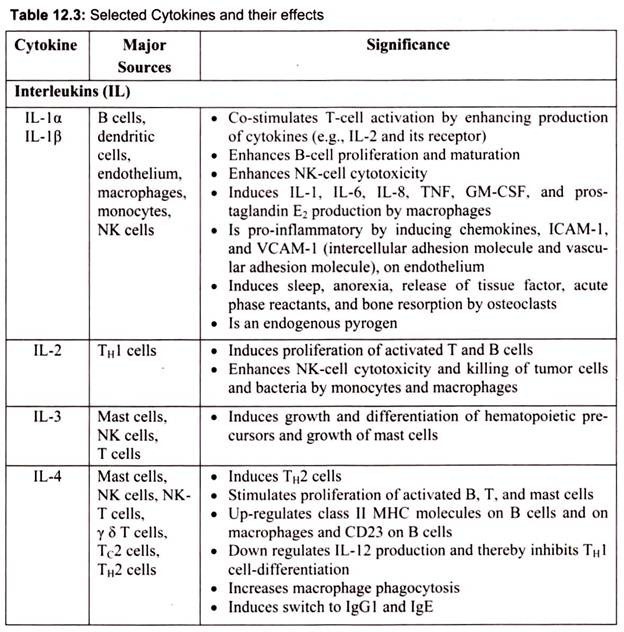
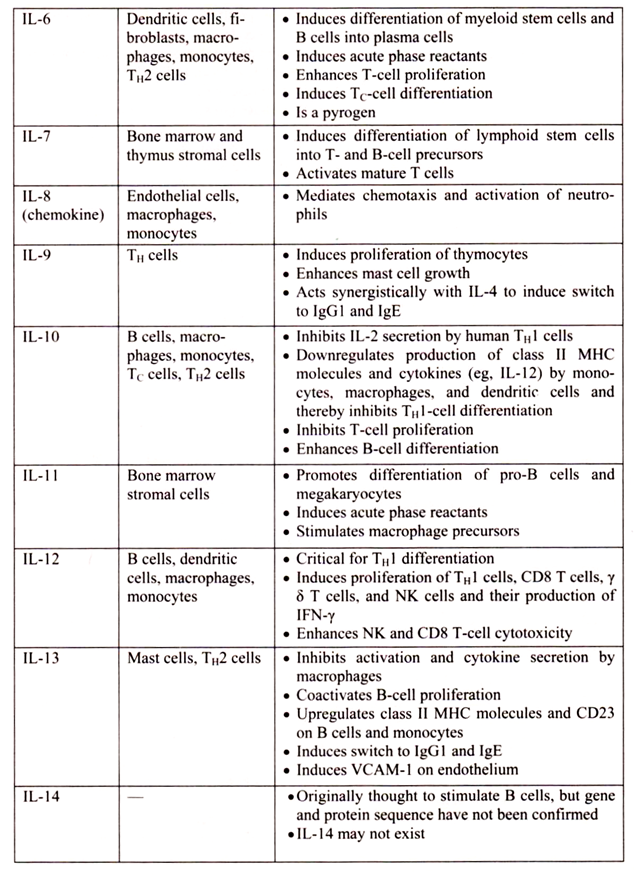
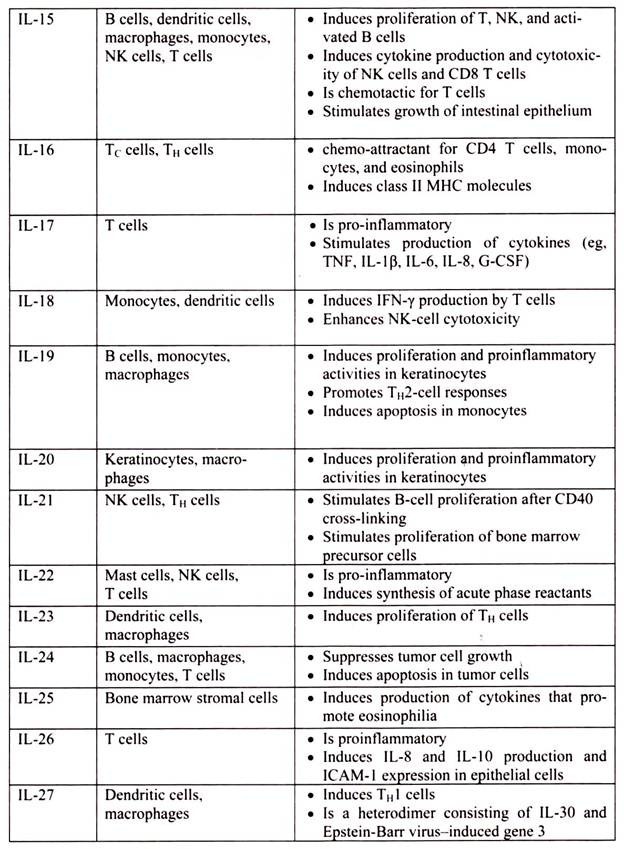
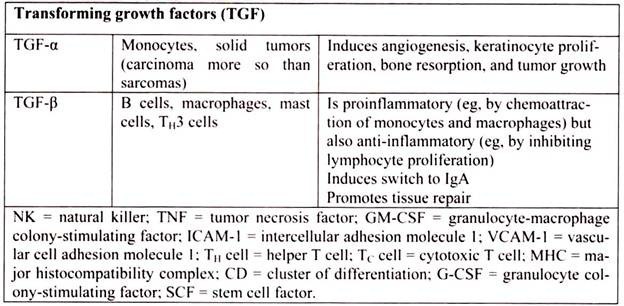
6. Essay on the Cytokines: Major Sources and Significance:
Cytokines are polypeptides secreted by immune and other cells when the cell interacts with a specific Ag, endotoxin, or other cytokines.
Main categories include:
i. IFNs (IFN-α, IFN-β, IFN-γ)
ii. TNFs (TNF-α, lymphotoxin-α, lymphotoxin-β)
iii. ILs
iv. Chemokines
v. TGFs
vi. Hematopoietic colony-stimulating factors (CSFs)
Although lymphocytes interaction with a specific Ag triggers cytokine secretion, cytokines themselves are not Ag-specific; thus, they bridge innate and acquired immunity and generally influence the magnitude of inflammatory or immune responses. They act sequentially, synergistically, or antagonistically and may act in an autocrine or paracrine manner.
Cytokines deliver their signals via cell surface receptors. For example, the IL-2 receptor consists of 3 chains- α, β, and γ. The receptor’s affinity for IL-2 is high if all 3 chains are expressed, intermediate if only the β and γ chains are expressed, or low if only the α chain is expressed. Mutations or deletion of the γ chain is the basis for X-linked severe combined immunodeficiency. Chemokines induces chemotaxis and migration of leukocytes. There are 4 subsets, defined by the number of intervening amino acids between the first 2 cysteine residues in the molecule. Chemokine receptors (CCR5 on memory T cells, monocytes/ macrophages, and dendritic cells; CXCR4 on resting T cells) act as co-receptors for entry of HIV into cells.
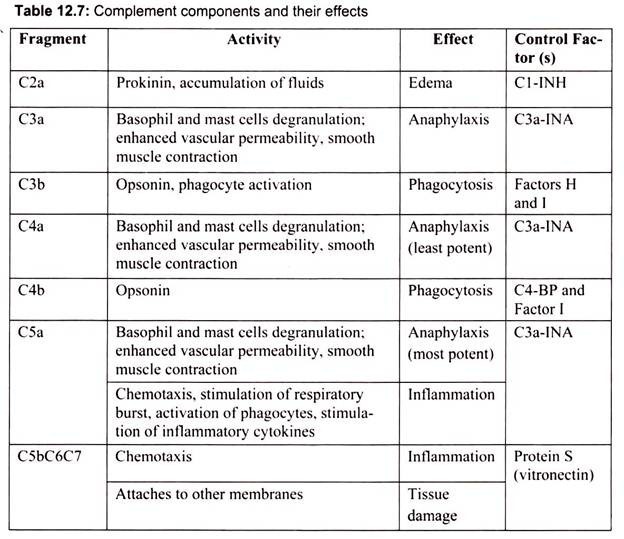
7. Essay on the Complement System:
The complement system is believed to play important functions in the innate immune response to infections, the generation of adaptive immune responses and the initiation of some autoimmune disorders. It is one of the oldest families of pattern recognition molecules and is involved in promoting opsonophagocytosis of bacterial and fungal pathogens, attraction and activation of phagocytes, clearance of immune complexes and apoptotic cells, and inducing inflammation and anaphylatoxins. The system consists of more than 30 soluble and cell- surface associated molecules responsible for initiation, effector functions, and regulation of three different enzyme cascades, termed the alternative-, classical- and mannose-binding lectin (MBL) pathways.
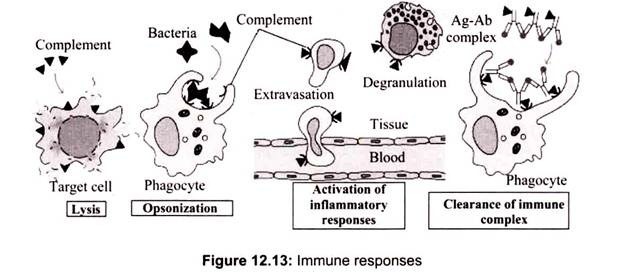
Historically, the term complement (C) was used to refer a heat-labile serum component that was able to lyse bacteria (activity is destroyed (inactivated) by heating serum at 56° C for 30 min). However, complement is now known to contribute to host defenses in other ways as well. Complement can opsonize bacteria for enhanced phagocytosis; it can recruit and activate various cells including polymorphonuclear cells (PMNs) and macrophages; it can participate in regulation of antibody responses and it can aid in the clearance of immune complexes and apoptotic cells. Complement can also have detrimental effects for the host; it contributes to inflammation and tissue damage and can trigger anaphylaxis.
Complement comprises over 20 different serum proteins that are produced by a variety of cells including, hepatocytes, macrophages and gut epithelial cells. Some complement proteins bind to immunoglobulins or to membrane components of cells. Others are proenzymes that, when activated, cleave one or more other complement proteins. Upon cleavage some of the complement proteins yield fragments that activate cells, increase vascular permeability or opsonize bacteria.
Some of the C5b67 complex formed can dissociate from the membrane and enter the fluid phase. If this were to occur it could then bind to other nearby cells and lead to their lysis. The damage to bystander cells is prevented by Protein S (vitronectin). Protein S binds to soluble C5b67 and prevents its binding to other cells (Table 12.7).
Pathways of Complement Activation:
The complement system is an enzyme cascade that helps defend against infection. Many complement proteins occur in serum as inactive enzyme precursors (zymogens); others reside on cell surfaces. The complement system bridges innate and acquired immunity by augmenting antibody (Ab) responses and immunologic memory, lysing foreign cells and clearing immune complexes and apoptotic cells. They have many biologic functions such as stimulation of chemotaxis and triggering of mast cell de-granulation independent of IgE).
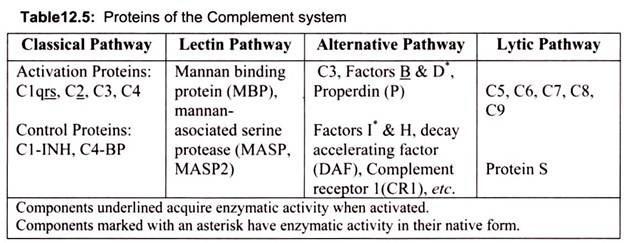
There are 3 pathways of complement activation:
i. Classical
ii. Lectin
iii. Alternative
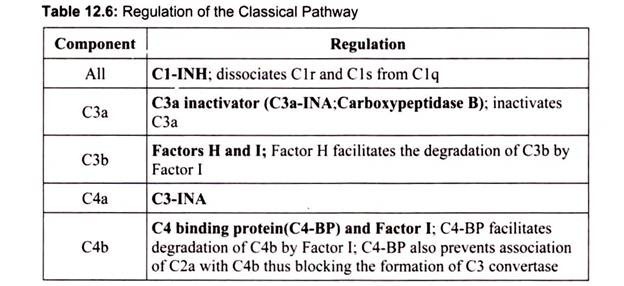
Classical pathway components are labeled with a C and a number (e.g., C1, C3), based on the order in which, they were identified. Alternative pathway components are often lettered (e.g., factor B, factor D) or named (e.g., properdin). Classical pathway activation is Ab- dependent, occurring when CI interacts with Ag-IgM or aggregated Ag-IgG complexes, or Ab-independent, occurring when polyanions (e.g., heparin, protamine, DNA and RNA from apoptotic cells), gram-negative bacteria, or bound C-reactive protein reacts directly with C1. This pathway is regulated by C1 inhibitor (C1-INH).
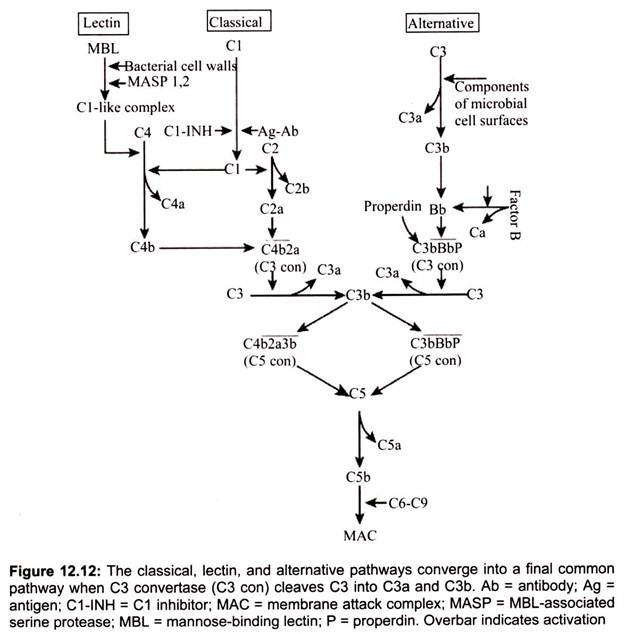
The three pathways all generate homologous variants of the protease, C3-convertase. C3 -convertase cleaves and activates C3, creating C3a and C3b and causing a cascade of further cleavage and activation events. C3b binds to the surface of pathogens leading to greater internalization by phagocytic cells. C5a is an important chemokine, which leads to the recruitment of inflammatory cells. C5b is initiates the membrane attack pathway which results in the membrane attack complex (MAC), consisting of C5b, C6, C7, C8, and polymeric C9. MAC is the cytolytic end product of the complement cascade; it forms a transmembrane channel which causes osmotic lysis of the target cell.
(a) Classical Pathway:
IgM or IgG antibody molecules, bound to the surface of micro-organisms, activate the complement system. The complement proteins actually recognize and bind the antibody on the surface of the pathogen. In this scenario the complement system could be considered as specific, but the antibody brings about the specificity so it merely complements the specific function of antibody. A series of proteins bind to the immune complex (C1, C2, C4), resulting in the formation of C3 convertase activity.
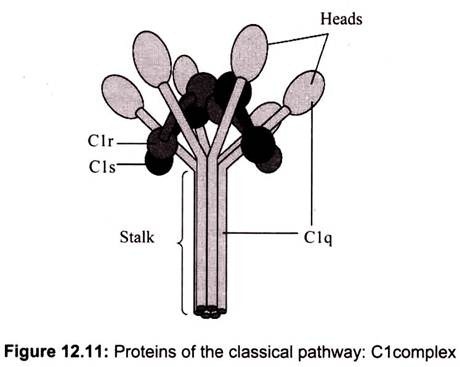
The classical pathway is triggered by activation of the CI-complex (which consist of one molecule C1q and two molecules C1r and C1s respectively), either by C1q binding to antibodies from classes M and G, complexed with antigens, or by binding C1q to the surface of the pathogen (Fig. 12.11). This binding leads to conformational changes in C1q molecule, which leads to the activation of two C1r (serine protease) molecules. Then they cleave C1s (another serine protease). The CI-complex now binds to and splits C2 and C4, producing C2a and C4b. The inhibition of C1r and C1s is controlled by C1-inhibitor. C4b and C2a bind to form C3-convertase (C4b2a complex). Production of C3-convertase signals the end of the classical pathway, but cleavage of C3 by this enzyme brings to the start of the alternative pathway.
(i) C1 Activation:
C1, a multi-subunit protein containing three different proteins (C1q, C1r and C1s), binds to the Fc region of IgG and IgM antibody molecules that have interacted with antigen. C1 binding does not occur to antibodies that have not complexed with antigen and binding requires calcium and magnesium ions. (In some cases CI can bind to aggregated immunoglobulin (e.g. aggregated IgG) or to certain pathogen surfaces in the absence of antibody). The binding of CI to antibody is via C1q and C1q must cross link at least two antibody molecules before it is firmly fixed. The binding of C1q results in the activation of C1r which in turn activates C1s. The result is the formation of an activated “C1qrs”, which is an enzyme that cleaves C4 into two fragments C4a and C4b.
(ii) C4 and C2 Activation (Generation of C3 Convertase):
The C4b fragment binds to the membrane and the C4a fragment is released into the microenvironment. Activated “C1qrs” also cleaves C2 into C2a and C2b. C2a binds to the membrane in association with C4b, and C2b is released into the microenvironment. The resulting C4bC2a complex is a C3 convertase, which cleaves C3 into C3a and C3b.
(iii) C3 Activation (Generation of C5 Convertase):
C3b binds to the membrane in association with C4b and C2a, and C3a is released into the microenvironment. The resulting C4bC2aC3b is a C5 convertase. The generation of C5 convertase is the end of the classical pathway. Several of the products of the classical pathway have potent biological activities that contribute to host defenses. Some of these products may also have detrimental effects if produced in an unregulated manner (Fig. 12.13).
If the classical pathway were not regulated there would be continued production of C2b, C3a, and C4a. Thus, there must be some way to regulate the activity of the classical pathway. Table 3 summarizes the ways in which the classical pathway is regulated (Table 12.6).
(b) Lectin Pathway:
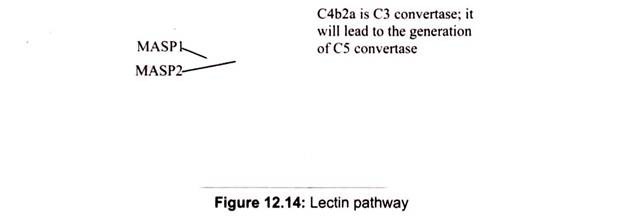
Lectin pathway activation is Ab-independent; it occurs when mannose-binding lectin (MBL), a serum protein, binds to mannose or fructose groups on bacterial cell walls, yeast walls, or viruses. This pathway otherwise resembles the classical pathway structurally and functionally.
It is initiated by the binding of mannose-binding lectin (MBL) to bacterial surfaces with mannose-containing polysaccharides (mannans). Binding of MBL to a pathogen results in the association of two serine proteases, MASP-1 and MASP-2 (MBL-associated serine proteases). MASP-1 and MASP-2 are similar to C1r and C1s, respectively and MBL is similar to C1q. Formation of the MBL/MASP-1/MASP-2 tri-molecular complex results in the activation of the MASPs and subsequent cleavage of C4 into C4a and C4b. The C4b fragment binds to the membrane and the C4a fragment is released into the microenvironment.
Activated MASPs also cleave C2 into C2a and C2b. C2a binds to the membrane in association with C4b and C2b is released into the microenvironment. The resulting C4bC2a complex is a C3 convertase, which cleaves C3 into C3a and C3b. C3b binds to the membrane in association with C4b and C2a and C3a is released into the microenvironment. The resulting C4bC2aC3b is a C5 convertase. The generation of C5 convertase is the end of the lectin pathway.
(c) Alternate Pathway:
Alternate pathway activation occurs when components of microbial cell surfaces (e.g., yeast walls, bacterial cell wall lipopolysaccharide (endotoxin) or Ig (e.g., nephritic factor, aggregated IgA) cleave small amounts of C3. This pathway is regulated by properdin, factor H, and decay-accelerating factor. The alternative pathway begins with the activation of C3 and requires Factors B and D and Mg++ cation, all present in normal serum.
(i) Amplification Loop of C3b Formation
In serum there is low level spontaneous hydrolysis of C3 to produce C3i. Factor B binds to C3i and becomes susceptible to Factor D, which cleaves Factor B into Bb. The C3iBb complex acts as a C3 convertase and cleaves C3 into C3a and C3b. Once C3b is formed, Factor B will bind to it and becomes susceptible to cleavage by Factor D. The resulting C3bBb complex is a C3 convertase that will continue to generate more C3b, thus amplifying C3b production. If this process continues unchecked, the result would be the consumption of all C3 in the serum. Thus, the spontaneous production of C3b is tightly controlled.
(ii) Control of the Amplification Loop
As spontaneously produced C3b binds to autologous host membranes, it interacts with DAF (decay accelerating factor), which blocks the association of Factor B with C3b thereby preventing the formation of additional C3 convertase. In addition, DAF accelerates the dissociation of Bb from C3b in C3 convertase that has already formed, thereby stopping the production of additional C3b. Some cells possess complement receptor 1 (CR1). Binding of C3b to CR1 facilitates the enzymatic degradation of C3b by Factor I.
In addition, binding of C3 convertase (C3bBb) to CR1 also dissociates Bb from the complex. Thus, in cells possessing complement receptors, CR1 also plays a role in controlling the amplification loop. Finally, Factor H can bind to C3b bound to a cell or in the in the fluid phase and facilitate the enzymatic degradation of C3b by Factor I. Thus, the amplification loop is controlled by either blocking the formation of C3 convertase, dissociating C3 convertase, or by enzymatically digesting C3b. The importance of controlling this amplification loop is illustrated in patients with genetic deficiencies of Factor H or I. These patients have a C3 deficiency and increased susceptibility to certain infections.
(iii) Stabilization of C Convertase by Activator (Protector) Surfaces:
C3b when bound to an appropriate activator of the alternative pathway, it will bind Factor B, which is enzymatically cleaved by Factor D to produce C3 convertase (C3bBb). However, C3b is resistant to degradation by Factor I and the C3 convertase is not rapidly degraded, since it is stabilized by the activator surface. The complex is further stabilized by properdin binding to C3bBb. Activators of the alternate pathway are components on the surface of pathogens and include LPS of Gram-negative bacteria and the cell walls of some bacteria and yeasts. Thus, when C3b binds to an activator surface, the C3 convertase formed will be stable and continue to generate additional C3a and C3b by cleavage of C3.
Generation of C5 Convertase:
Some of the C3b is generated by the stabilized C3 convertase on the activator surface associates with the C3bBb complex to form a C3bBbC3b complex. This is the C5 convertase of the alternative pathway. The generation of C5 convertase is the end of the alternative pathway. The alternative pathway can be activated by many Gram-negative (most significantly, Neisseria meningitidis and N. gonorrhoea), some Gram-positive bacteria and certain viruses and parasites, and results in the lysis of these organisms. Thus, the alternative pathway of C activation provides another means of protection against certain pathogens before an antibody response is mounted. A deficiency of C3 results in an increased susceptibility to these organisms. The alternate pathway may be the more primitive pathway and the classical and lectin pathways probably developed from it.
Membrane Attack (Lytic) Pathway:
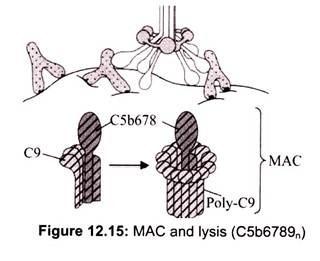
C5 convertase from the classical (C4b2a3b), lectin (C4b2a3b) or alternative (C3bBb3b) pathway cleaves C5 into C5a and C5b. C5a remains in the fluid phase and the C5b rapidly associates with C6 and C7 and inserts into the membrane. Subsequently C8 binds, followed by several molecules of C9. The C9 molecules form a pore in the membrane through which the cellular contents leak and lysis occurs. Lysis is not an enzymatic process; it is thought to be due to physical damage to the membrane. The complex consisting of C5bC6C7C8C9 is referred to as the membrane attack complex (MAC).
C5a generated in the lytic pathway has several potent biological activities. It is the most potent anaphylotoxin. In addition, it is a chemotactic factor for neutrophils and stimulates the respiratory burst in them and it stimulates inflammatory cytokine production by macrophages. Its activities are controlled by inactivation by carboxypeptidase B (C3-INA).
8. Essay on the Major Histocompatibility Complex:
The Major Histocompatibility Complex (MHC) is a set of molecules displayed on cell surfaces that are responsible for lymphocyte recognition and “antigen presentation”. The MHC molecules control the immune response through recognition of “self’ and “non-self” and, consequently, serve as targets in transplantation rejection. The Class I and Class II MHC molecules belong to a group of molecules known as the Immunoglobulin Supergene Family (Fig. 12.16), which includes immunoglobulins, T-cell receptors, CD4, CD8, and others.
The major histocompatibility complex is encoded by several genes located on human chromosome 6- Class I molecules are encoded by the BCA region while class II molecules are encoded by the D region. A region between these two on chromosome 6 encodes class III molecules, including some complement components.
Gene products encoded in the Major Histocompatibility Complex (MHC) were first identified is being important in rejection of transplanted tissues. Furthermore, genes in the MHC were found to be highly polymorphic (i.e. in the population there were many different allelic forms of the genes). Studies with inbred strains of mice showed that genes in the MHC were also involved in controlling both humoral and cell-mediated immune responses.
For example, some strains of mice could respond to a particular antigen but other strains could not and these strains differed only in one or more of the genes in the MHC. Subsequent studies showed that there were two kinds of molecules encoded by the MHC – Class I molecules and class II molecules. Class I molecules were found on all nucleated cells whereas class II molecules were found only on antigen presenting cells, (APCs) which included dendritic cells, macrophages, B cells and a few other types.
It was not until the discovery of how the T cell receptor (TCR) recognizes antigen that the role of MHC genes in immune responses was understood. The TCR was shown to recognize antigenic peptides in association with MHC molecules. T cells recognize portions of protein antigens that are bound non-covalently to MHC gene products. Cytotoxic T cells (Tc) recognize peptides bound to class I MHC molecules and helper T cells (Th) recognize peptides bound to class II MHC molecules. The mechanism by which the TCR, MHC gene products and antigen interact has been elucidated by the three dimensional structures of MHC molecules and the TCR determined by X-ray crystallography.
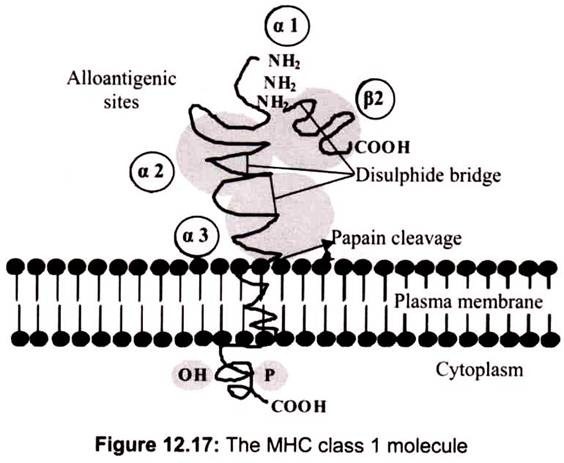
A. Structure of Class I MHC Molecules:
Class I molecules are composed of two polypeptide chains; one encoded by the BCA region and another (β2-microglobulin) that is encoded elsewhere. The MHC-encoded polypeptide is about 350 amino acids long and glycosylated, giving a total molecular weight of about 45 kDa. This polypeptide folds into three separate domains called alpha-1, alpha-2 and alpha-3. β2-microglobulin is a 12 kDa polypeptide that is non-covalently associated with the alpha-3 domain. Between the alpha-1 and alpha-2 domains lies a region bounded by a beta-pleated sheet on the bottom and two alpha helices on the sides. This region is capable of binding (via non-covalent interactions) a small peptide of about 10 amino acids. This small peptide is “presented” to a T-cell and defines the antigen “epitope” that the T-cell recognizes.
Class I MHC molecules are composed of two polypeptide chains, a long α chain and a short β chain called β2-microglobulin. The a chain has four regions. First, a cytoplasmic region, containing sites for phosphoylation and binding to cytoskeletal elements. Second, a transmembrane region containing hydrophic amino acids by which the molecule is anchored in the cell membrane. Third, a highly conserved α3 immunoglubilin-like domain to which CD8 binds. Fourth, a highly polymorphic peptide binding region formed from the α1 and α2 domains. The β2- microglobulin associates with the a chain and helps maintain the proper conformation of the molecule (Fig. 12.17).
The α1 and α2 domains of the class I MHC molecules, which comprise the peptide binding region is found to be the most variable region. The structure of the peptide binding groove, revealed by X-ray crystallography, shows that the groove is composed of two α helices forming a wall on each side and eight β-pleated sheets forming a floor. The peptide is bound in the groove and the residues that line the groove make contact with the peptide.
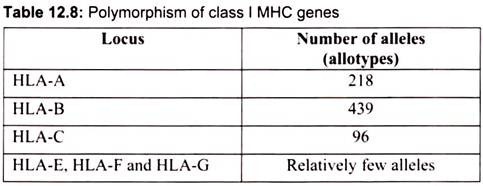
These are the residues that are most polymorphic. The groove will accommodate peptides of approximately 8-10 amino acids long. Whether a particular peptide will bind to the groove will depend on the amino acids that line the groove. Because class I molecules are polymorphic, different class I molecules will bind different peptides. Each class I molecule will bind only certain peptides and will have a set of criteria that a peptide must have in order to bind to the groove. Within the MHC there are 6 genes that encode class I molecules HLA-A, HLA -B, HLA-C, HLA-E, HLA-F and HLA-G. Among these HLA-A, HLA -B, and HLA-C are the most important and also most polymorphic. Table 12.8 shows the degree of polymorphism at each of these loci.
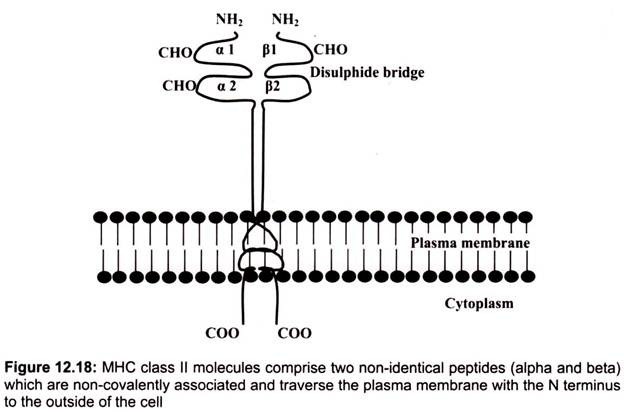
B. Structure of Class II MHC Molecules:
Class II MHC molecules are composed of two polypeptide chains an α and a β chain of approximately equal length. Both chains have four regions- first, a cytoplasmic region containing sites for phosphoylation and binding to cytoskeletal elements; second, a transmembrane region containing hydrophic amino acids by which the molecule is anchored in the cell membrane, third, a highly conserved α2 domain and a highly conserved β2 domain to which CD4 binds and fourth, a highly polymorphic peptide binding region formed from the al and 01 domains.
The α1 and β2 domains of the class II MHC molecules, which comprise the peptide binding region are found to be the most variable regions. The structure of the peptide binding groove, revealed by X-ray crystallography, shows that, like class I MHC molecules, the groove is composed of two a helices forming a wall on each side and eight β-pleated sheets forming a floor. Both the al and pi chain contribute to the peptide binding groove. The peptide is bound in the groove and the residues that line the groove make contact with the peptide.
These are the residues that are the most polymorphic. The groove of Class II molecules is open at one end so that the groove can accommodate longer peptides of approximately 13- 25 amino acids long with some of the amino acids located outside of the groove. Whether a particular peptide will bind to the groove will depend on the amino acids that line the groove. Because class II molecules are polymorphic, different class II molecules will bind different peptides. Like class I molecules, each class II molecule will bind only certain peptides and will have a set of criteria that a peptide must have in order to bind to the groove.
Within the MHC there are 5 loci that encode class II molecules, each of which contains a gene for an a chain and at least one gene for a P chain. The loci are designated as HLA-DP, HLA-DQ, HLA-DR, HLA-DM, and HLA-DO. Among these, HLA-DP, HLA -DQ and HLA-DR are the most important and polymorphic in nature.
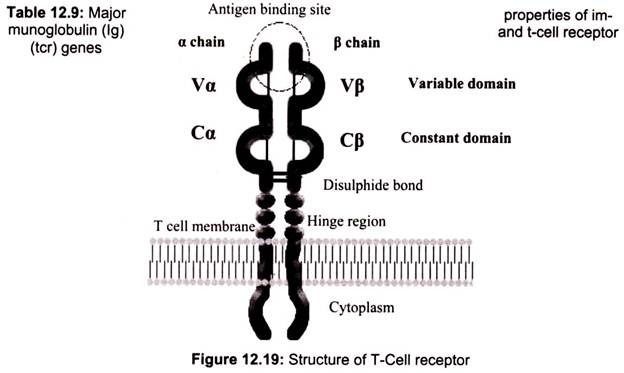
9. Essay on the T Cell Receptor (TCR):
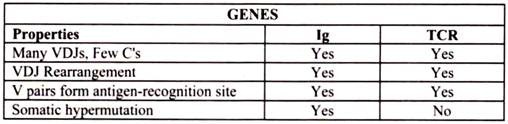
The TCR is a heterodimer composed of one α and one β chain of approximately equal length. Each chain has a short cytoplasmic tail but it is too small to be able to transduce an activation signal to the cell. Both chains have a transmembrane region comprised of hydrophobic amino acids by which the molecule is anchored in the cell membrane. Both chains have a constant region and a variable region similar to the immunoglobulin chains.
The variable region of both the chains contains hypervariable regions that determine the specificity for antigen. Each T cell bears a TCR of only one specificity (i.e. there is allelic exclusion). The T cell receptor heterodimer comprises of two transmembrane glycoproteins, the alpha and beta chains. There are two domains in the external part of each chain and these resemble immunoglobulin variable and constant regions. There are sugar chains on each domain. There is a short sequence similar to the immunoglobulin hinge region that connects the immunoglobulin-like domains to the transmembrane sequence.
This contains cysteines that form a disulfide bridge. The hydrophobic transmembrane helical structures are unusual in that they contain positively charged amino acids (basic amino acids). The alpha chain has two positively charged residues while the beta chain has one. The T cell receptor (TCR) is a complex of integral membrane proteins that participates in the activation of T cells in response to the presentation of antigen. Specific recognition and binding by the clonotype-specific a/b heterodimer leads to activation of transcription and commitment of the T cell to CD4+ or CD8+ fate. This activation involves other subunits of the receptor complex as well as other membrane-associated molecules that couple the extracellular liganding event to downstream signaling pathways such as protein phosphorylation, the release of inositol phosphates and the elevation of intracellular calcium levels.
The germline genes for the TCR β genes are composed of V, D and J gene segments that rearrange during T cell development to produce many different TCR β chains. The germline genes for the TCR α genes are composed of V and J gene segments which rearrange to produce α chains. The specificity of the TCR is determined by the combination of α and β chains.
There is a small population of T cells that express TCRs that have γ and δ chains instead of α and β chains. These gamma/delta T cells predominate in the mucosal epithelium and have a repertoire biased toward certain bacterial and viral antigens. The genes for the δ chains have V, D and J gene segments whereas the genes for the γ chains have only V and J gene segments but the repertoire is considerably smaller than that of the alpha/beta T cells. The γ/δ T cells recognize antigen in an MHC-independent manner unlike the α/β T cells.
(i) TCR and CD3 Complex:
The TCR is closely associated with a group of 5 proteins collectively called CD3 complex. The CD3 complex is composed of one γ, one δ, two ε and 2 ξ chains. The CD3 is actually a complex of 5 polypeptides. Gamma, delta, and epsilon occur as 2 dimers [gamma- epsilon and delta-epsilon] complexed with either a homodimer of two zeta chains or a heterodimer of zeta and eta chains.
Zeta and eta are encoded by the same gene and only differ in their carboxyl terminus. The two forms are generated by differential RNA splicing of the primary transcript. All of the proteins of the CD3 complex are invariant and they do not contribute to the specificity in any way. The CD 3 complex is necessary for cell surface expression of the TCR during T cell development. In addition, the CD3 complex transduces activation signals to the cell following antigen interaction with the TCR.
Short length of the cytoplasmic tail of the alpha & beta chains suggests that they are unsuitable for signal transduction. CD3 functions in this regard. The TcR exists on the membrane as a molecular complex with CD3. Mutation in either the CD3 or the TcR results in loss of the entire complex from the membrane. The transmembrane domain of all CD3 polypeptides contains a -ve charged aspartic acid. Correspondingly, there are either 1 or 2 + charged amino acids in the transmembrane domain of each TcR chain. The alpha chain contains a lysine and an arginine while the beta chain contains a single lysine. Once the TcR recognizes an antigen-MHC complex, the associated CD3 complex is thought to transmit a signal to the cell interior that contributes to cellular activation.
Monoclonal antibody specific for CD3 bypasses the antigen-specific T cell receptor. Following activation, several of the CD3 polypeptides are phosphorylated at tyrosine or serine residues. Phosphorylation is thought to lead to the activation of second messengers involved in T cell activation. The phosphorylation occurs at sequences within the cytoplasmic domains of the CD3 glycoproteins which are termed ITAMS (immuno-receptor tyrosine-based activation motifs). These ITAM sequences (also found in the cytoplasmic domains of the Iga /Igb heterodimers of the B cell receptor complex) interact with tyrosine kinases (the enzymes which catalyze phosphorylation at tyrosine or serine).
(ii) CD4 and CD8 Accessory Molecules:
CD4 and CD8 are associated in the membrane with the TCR. Both CD4 and CD8 function as adhesion molecules; CD4 binds to Class II MHC molecules and CD8 binds to Class I MHC molecules. Binding of the TCR to the peptide/MHC complex is greatly augmented if CD4 or CD8 are assisting. Their cytoplasmic domains may also allow for signal transduction to occur. The signal-transduction property of CD4 and CD8 is mediated through their cytoplasmic domains. Both CD4 and CD8 are non-covalently associated with the protein kinase Lck.
Variety of other membrane molecules plays an important accessory role in antigen recognition and T cell activation. In addition to CD4 and CD8, T cells possess several other accessory molecules including LFA-1 and CD28. LFA-1 is an adhesion molecule which strengthens the interaction of the T cell with an APC or target cell. LFA-1 is an integrin which binds to ICAM-1 (inter-cell adhesion molecule on the APC or target cell. CD28 on Th cell binds to B7 on an APC or target cell. This interaction functions as a co-stimulatory signal leading to activation of the T lymphocyte. Without this co-stimulatory signal, the T lymphocyte experiences energy (non-responsive, basically the opposite of activation).
The interactions between the TCR and MHC molecules are not very strong.
Accessory molecules are necessary to help stabilize the interaction. These include:
(1) CD4 binding to Class II MCH, ensures that Th cells only interact with APCs;
(2) CD8 binding to class I MHC, which ensures that Tc cells can interact with target cells;
(3) CD2binding to LFA-3 and 4) LFA-1 binding to ICAM-1.
The accessory molecules are invariant and do not contribute to the specificity of the interaction, which is solely determined by the TCR. The expression of accessory molecules can be increased in response to cytokine, which is one way that cytokines can modulate immune responses. In addition to accessory molecules which help stabilize the interaction between the TCR and antigen in association with MHC molecules, other molecules are also needed for T cell activation. Two signals are required for T cell activation; one is the engagement of the TCR with Ag/MHC and the other signal comes from the engagement of co-stimulatory molecules with their ligands.
One of the most important (but not the only) co-stimulatory molecule is CD28 on T cells which must interact with B7-1 (CD80) or B7-2 (CD81) on APCs. Like accessory molecules the co-stimulatory molecules are invariant and do not contribute to the specificity of the interaction. The multiple interactions of TCR with Ag/MHC and the accessory and co-stimulatory molecules with their ligands have been termed the “immunological synapse.”
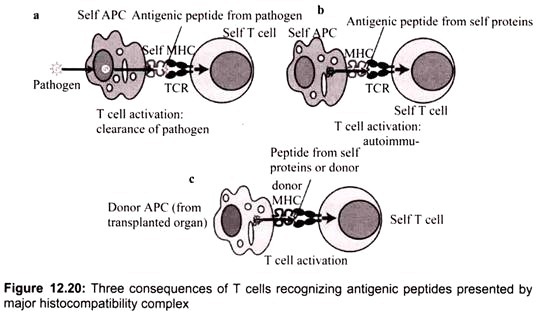
There are three consequences of T cells recognizing antigenic peptides presented by major histocompatibility complex (MHC) molecules on antigen-presenting cells:
(a) During an infection, T cells recognize antigenic peptide fragments of the pathogen that are presented on the surface of MHC molecules. This recognition is mediated by the T-cell receptor (TCR) and stabilized by additional cell-surface molecules,
(b) The nature of T-cell development means that potentially self-reactive T cells are produced that can respond to self MHC molecules, even in the absence of infection,
(c) In transplantation, T-cell activation in response to foreign (allogeneic) MHC molecules on the surface of donor antigen-presenting cells (APCs) induces destructive immune responses (Fig. 12.20).
10. Essay on the B- Cell Receptor:
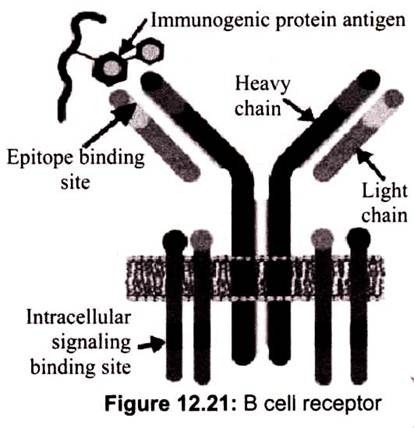
B-Lymphocytes and T-Lymphocytes are the most abundant lymphocytes. B-Cells are produced and mature in the bone marrow and are specific for a particular antigen. The specificity of binding resides in the BCR (B-Cell receptor) for antigen. They are integral membrane proteins. They are present in thousands of identical copies exposed at the cell surface. They are made before the cell ever encounters an antigen. B-Cell receptor complex usually consist of an antigen-binding subunit (the membrane immunoglobulin or Mlg), which is composed of two IgHs (Immunoglobulin Heavy Chains) and two IgLs (Immunoglobulin Light Chains), and a signaling subunit, which is a disulfide-linked heterodimer of Ig-Alpha (CD79A) and Ig -Beta (CD79B) proteins.
For the heavy chains of BCRs (antibodies), the gene segments are the 51 VH segments, each of which encodes most of the N-terminal of the antibody, including the first two (but not the third) hyper variable region, 25 DH (=“diversity”) gene segments, which encode part of the third hyper variable region, 6 JH (=“joining”) gene segments, encoding the remainder of the V region of the BCR (including the remainder of the third hyper variable region) and 9 CH gene segments, which encode the C region of the BCR (and the antibody derived from it).
All of these gene segments are clustered in a complex locus on chromosome 14. During the differentiation of the B-Cell (and long before any possible encounter with an antigen), the DNA in this locus is cut and recombined to make an intact gene for the heavy chain. This gene can then be transcribed into mRNA, which is, in turn, translated into the heavy polypeptide chain. All isotypes of mlg have very short cytoplasmic tails. Both mlgM and mlgD have a cytoplasmic domain, which are only 3 amino acids in length. The cytoplasmic tails of mlg are too short to be able to associate with intracellular signaling molecules.
Since mlg is always associated with the Ig-alpha/Ig-beta heterodimer collectively forming B-Cell receptor complex (BCR), two molecules of this heterodimer associate with one mlg to form a single BCR. The Ig-alpha/Ig-beta heterodimer carries out the signal transducing function of the complex. The Ig-alpha chain has a long cytoplasmic domain containing 61 amino acids while the Ig-beta chain has a long cytoplasmic domain containing 48 amino acids.
BCR have a unique binding site. This site binds to a portion of the antigen called an antigenic determinant or epitope. The binding depends on complementarity of the surface of the receptor and the surface of the epitope. The binding occurs by non-covalent forces. In the absence of specific antigen, mature B-Cells survive in the peripheral circulation for only a few days. Cells which do not encounter antigen within this period of time undergo apoptosis. This is necessary in order to maintain an optimal circulation of B-lymphocytes in the peripheral circulation. The receptor on the cell surface of B-lymphocytes functions to transmit intracellular signals that regulate cell growth and differentiation and it binds to antigen for the generation of the immune response.
Most B-Cell antigens are T dependent. In other words, the B-Cell requires direct contact by TH lymphocytes as well as exposure to TH lymphocyte cytokines in order to be fully activated. There are a few T independent antigens. One of the best-known examples of a T independent antigen is LPS (Lipopolysaccharide). At low concentrations, LPS stimulates the production of specific antibodies (LPS-specific) but at high concentrations it can cause the polyclonal activation of B-Cells. The polyclonal activation of B-Cells leads to the proliferation and differentiation of large numbers of B-Cells, regardless of their antigen specificity.
11. Essay on the Antigens, Mitogens and Immunomodulating Substances:
Antigens:
Antigen, could be any substance that when introduced into the body stimulates the production of an antibody. Antigens include toxins, bacteria, foreign blood cells, and the cells of transplanted organs. Based on the immunological properties, antigens can be categorized as immunogenic, antigenic, allergenic and tolerogenic. Immunogenic substances are those which can induce a humoral or cell-mediated immune response.
B cells + antigen → plasma cells + memory cells
T cells + antigen → T effector cells + memory cells
In contrast, some small molecules referred to as haptens are not capable by themselves of inducing a specific immune response. In simple terms, they lack immunogenicity. In order to induce immune response, the hapten requires to be attached to a carrier molecule (usually a serum protein such as albumin). The hapten molecule then acts as a determinant of antigenic specificity and is referred to as an antigenic determinant. Classical haptens include di- and trinitrophenol (DNP and TNP), dimethyl aminonaphthalene sulfonate (dansyl), and a number of toxins, including urushiol, which is the toxin found in poison ivy.
Carriers are macromolecules that bind haptens and enable them to induce an immune response. Most carriers are secretory proteins or proteins on the cell surface, where cells of the immune system can reach them. First, step in the induction of immune response by a hapten is binding to its carrier. The carrier, normally ignored by the immune system now has a different surface structure that can act as an antigen (the hapten on its own is too small).
Depending on the type of immune cell that recognizes the antigen, two pathways can be distinguished:
(a) The B-cell mediated pathway. It leads to the production of immunoglobulins against the hapten- carrier compound. A great variety of haptens and carriers have been shown to act this way.
(b) The T-cell mediated pathway. It leads to cytotoxic T-cell activity and to the recruitment of inflammatory cells. Usually, the carrier is a MHC molecule recognized by the T-cell receptor. In contact dermatitis, a well-resolved subgroup of this pathway, the haptens travel a long way with Langerhans cells before being checked by T cells in the lymphatic system.
Allergenic substances are those which have the ability to induce various types of allergic responses. Allergens are immunogens that tend to activate specific types of humoral or cell- mediated responses. Tolerogenic substances are those which can induce specific immunologic non-responsiveness in either the humoral or cell-mediated branch.
Mitogens:
Mitogens are agents that can induce cell division in a high percentage of T or B cells. Unlike immunogens, which activate only lymphocytes bearing specific receptors, mitogens activate many clones of T or B cells regardless of their antigen specificity. Because of this ability, mitogens are known as polyclonal activators. Various diverse agents function as mitogens. Many common mitogens are proteins (called lectins) that are derived from plants and bind sugars. Lectins recognize different glycoproteins on the surface of various cells, including lymphocytes.
Their binding often leads to agglutination, or clustering of the cells, followed by cellular activation. Some mitogens preferentially activate B cells, others activate T cells, and some activate both. Three commonly used lectins with mitogenic activity are concana- valin A (Con A), phyto-hemagglutinin (PHA) and pokeweed mitogen. Each of these binds to carbohydrate residues in glycoproteins and is able to crosslink glycoproteins on the surface of cells.
The lipopolysaccharide (LPS) component of the gram-negative bacterial cell wall functions as a B-cell mitogen, Con A and PHA are T-cell mitogens, while pokeweed mitogen acts as both. Among the group of T cell mitogens, an unusual group of substances, known as superantigens, is found to be most potent.
Immunomodulating Substances:
A variety of substances, when used as adjuvants, in combination with specific vaccines, enhance immunity levels above those which the vaccine elicits by itself, Agar, tapioca, lecithin, and some other substances show this potentiating effect. Certain peptides of animal origin are potent stimulants (or adjuvants) of the immune system whereas others act as inhibitors and in some cases the same peptide stimulates under certain conditions and on the contrary inhibits under other conditions the immune response.
These peptides with dual function are called immunomodulators. Good examples of immunomodulator peptides include interferon, interleukin, muramyl peptides of microbial origin (e.g., murabutide and lipopeptides which attack phagocytic cells and T-lymphocytes), several peptides of animal origin, including thymic hormones (e.g., thymulin promotes many T-cell functions and inhibits the generation of cytotoxic T-lymphocytes), tuftsin (stimulates phagocytic cells, pinocytosis, phagocytosis, motility, and antigen processing), peptides derived from fibrinogen, and certain peptides from colostrums and from milk.
Furthermore, some neuropeptides (neuroendocrine hormones) are thought to play an essential role as chemo signals from the central nervous system to the immune system. Somatostatin is a tetradecapeptide, isolated from the hypothalamus that inhibit the secretion of growth hormone. It is present in neural and gastrointestinal tissues and can also inhibit secretion from several endocrine and exocrine glands. Specific functional receptors for a variety of neuropeptides have been demonstrated in diverse cell populations of the immune system.
12. Essay on the Vaccines:
Recent advances in immunology have led to the development of new and promising vaccine strategies. Knowledge of differences in epitopes recognized by T cells and B cells has enabled immunologists to design vaccines to maximize activation of the humoral or cell- mediated branch of the immune system. Genetic engineering techniques facilitate development of vaccines which maximize the immune response to selected epitopes.
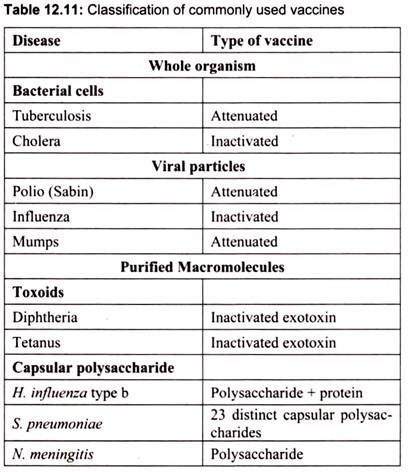
Table 12.11 indicates some currently used vaccines:
Vaccines work by priming the immune system to swiftly destroy the disease causing agents before they can multiply enough to cause symptoms. To date, this priming has been achieved by presenting the immune system with whole viruses or bacteria that have been killed or attenuated (made too weak to proliferate much). The immune system responds to this vaccine as if it was under attack by a fully potent antagonist and mobilizes its forces to destroy the foreign body. Memory cells are then left behind on alert, ready to unleash whole armies of defenders if the real pathogen ever finds its way into the body.
Classical vaccines pose a small risk in that the killed or attenuated microorganism may sometimes spring back to life, causing the disease the vaccine was meant to prevent. For this reason, ‘subunit’ vaccines (which contain no genes, just proteins derived from them) are now favored since they reduce this risk. They are, however, often not as effective as live vaccines. Subunit vaccines are also expensive, because they are produced in cultures of bacteria or animal cells and have to be purified and refrigerated.
Many researchers hope to develop edible vaccines which are similar to subunit preparations, containing only the genes coding for certain antigens, not the whole virus or bacterium. One main hurdle to be overcome here is that the antigens could be degraded in the stomach before having time to act. Typical subunit vaccines have to be delivered by injection precisely because of this. Researchers working on an edible hepatitis B vaccine suggest that oral doses may need to be 10-100 times higher than an injectable dose to elicit a comparable immune response.
One of the aims of the edible vaccine proponents is to reduce immunization costs. They feel that edible vaccines would be far cheaper than current injectable vaccines since they would not have to undergo the expensive purification and refrigeration of traditional vaccines, and shipping costs may be less. However, shipping costs may not necessarily be significantly reduced, and edible vaccines may still require refrigeration. Even if edible vaccines are cheaper, it does not follow that this will lead to increased vaccination coverage, since the cost of the vaccine is only a small part of the whole package.
Producing stable and reliable amounts of vaccines in plants is complicated by the fact that tomatoes and bananas do not come in standard sizes. In many developing countries, stringent quality control for standard drugs does not exist. People could ingest too much of the vaccine, which could be toxic, or too little — which could lead to disease outbreaks among populations believed to be immune.
Oral vaccines are also more difficult to formulate than injectables (for example, the oral polio vaccine is more convenient but less effective than the injectable one). The vaccines are likely to need cofactors (adjuvants) such as cholera toxin to enhance their uptake and increase their effectiveness; in addition, new vaccines have to be tested worldwide, since their effectiveness is not uniform in different contexts.
Ideal Vaccine:
An ideal vaccine should be safe, efficacious, cheap, easily administrate (e.g. orally), and thermally stable. It should confer long-term immunity. A single vaccine should preferably protect against several locally important infectious diseases, i.e. should be multivalent. Some of the currently available viral vaccines meet many of these requirements. The efficacy of a vaccine depends primarily upon the nature and persistence of the induced immune response. Some understanding has come from the study of model viral-host systems, such as the murine influenza model.
Four stages are readily identified and include:
(1) Prevention of infection,
(2) Limitation of viral replication,
(3) Recovery from infection, and
(4) Generation of memory cells.
Vaccines function by stimulating adaptive immune response. They are usually administered before exposure to the wild-type agent has occurred. The time interval may be weeks, months or years. The vaccine should stimulate B-cells, T-helper (Th) cells, and Tc cells. In the case of an antigenic ally stable infectious agent, neutralizing antibody is the immune parameter of most importance.
The four requirements of an ideal vaccine are:
(1) Activation of antigen-presenting cells to initiate antigen processing and production of interleukins;
(2) Activation of both T- and B- cells to give a high yield of memory cells;
(3) Due generation of antibody to two or three B- cell epitopes and of Th and Tc cells to several epitopes; and
(4) Persistence of antigen, probably mainly on follicular dendritic cells in lymphoid tissue resulting in the continuing presence of antibody.