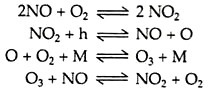Read this essay to learn about Air Pollution in India. After reading this essay you will learn about: 1. Meaning of Air Pollution 2. Causes of Air Pollution 3. Sources 4. Effects 5. Consequence 6. Air Pollution 7. Control 8. How to Reduce Air Pollution?.
Essay Contents:
- Essay on the Meaning of Air Pollution
- Essay on the Causes of Air Pollution
- Essay on the Sources of Air Pollution
- Essay on the Effects of Air Pollution
- Essay on the Consequence of Air Pollution
- Essay on the Air Pollution in India
- Essay on the Control of Air Pollution
- Essay on How to Reduce Air Pollution
Essay # 1. Meaning of Air Pollution:
Air pollution may be defined as imbalance in the quality of air so as to cause ill effects. According to Maxwell, “our enormously accelerated abuse of the atmosphere has become a health hazard and a threat to life, damaging both plants and animals in areas polluted with poisonous fumes, dust and smoke”.
The different types of pollutants are continuously introduced into the atmosphere and are removed by natural process of cleaning. But when pollution exceeds the atmosphere’s self purifying capacity, accumulation of pollutants occurs causing serious hazards for environment and other organisms including humans.
The following are the main reasons for the increasing rate of our pollution:
(i) Poisonous gases and other particles emitted from industries without any treatment.
(ii) Heavy increase in the number of automobiles and their emission.
(iii) Increased use of chemicals and petro-chemicals.
(iv) Population concentration in cities.
(v) Fast rate of deforestation.
(vi) Tests of experiments of atomic weapons.
(vii) Tests of chemical and bio chemical weapons.
(viii) Space research and satellite wastes.
(ix) Unorganized mining and traditional practices of the use of fuel wood etc.
Certain natural events such as volcanic eruption, dust storms etc., are also a cause of air pollution. In order to understand the nature of air pollution it is necessary to know the various sources of pollution.
Essay # 2. Causes of Air Pollution:
a. Pollution by Automobiles:
The automobiles is man’s greatest achievement in minimising distances. The number of automobiles is increasing day by day and has become a cause of air pollution and degradation of the environment.
The automobile illustrates how the free market principle, in this case the principle of freedom to select one’s method of transportation, may conflict with the aim of maintaining an environment congenial to people. For a long time, the growth of the automobile was regarded as an indication of prosperity and a good standard of living.
The expansion in individual transport now promoted materially and psychologically from both a political and commercial point of view and this led to the present situation. The automobile, with its internal combustion engine, emits poisonous gases which are harmful to human health and is the most serious pollution problem of the technological age.
The exhaust emission is a major source of air pollution in an automobile. Evaporative losses from fuel tank and carburettor and losses from the crank case, particulates from road surface, rubber tyres, brake linings and clutch plates also contribute significantly.
If oxidation were complete, water and carbon dioxide would be the only products produced from the combustion of petrol in an internal combustion engine. Neither of these products is considered to be a pollutant. But in practice it is difficult to achieve complete oxidation and carbon monoxide is formed in considerable quantities. Also a part of the fuel remains unchanged and some other compounds.
Apart from these products of incomplete combustion most petrols contain anti-knock agents which contain lead. The lead usually added to gasoline is the organic compound ‘tetra ethyl lead’ (TEL) which is extremely poisonous.
In addition, the conditions in the combustion chamber favour the oxidation of the nitrogen in air so that oxides of nitrogen are also formed in the engine. Further under favourable conditions the products emitted by vehicles can react with one another to produce unpleasant secondary pollutants.
It becomes clear from the above analysis that the problem of air pollution is increasing with the growth and expansion of industries and automobiles. It is high time that all of us should know the harmful effects of air pollution and also evolve technology to control it.
b. Industrial Emission:
The rapid rate of industrialisation has resulted in more and more air pollution. Various industrial processes release almost all types of pollutants into the air. Some industries like cement, iron and steel, fertilizer, petrochemical etc. are of great concern because of the difficulty in controlling the emission of pollutants from them. Acid ram has become a great threat to the environment.
The use of solvents is increasing with the growing use of paints, spray, polish etc. Due to the presence of hydrocarbons in these materials, air pollution is caused which is dangerous for health. Similarly, spray of pesticides in agriculture is also responsible for air pollution even in rural areas.
c. Chemical Industry:
Chemical reactions do not always continue to completion, so that in addition to the desired product, by -products occur, which on cost grounds are often released by the chemical industry into the atmosphere or discharged into the drainage system.
The spectrum of products manufactured by the chemical industry is extraordinary wide-ranging; inorganic substances such as sulphuric, nitric, hydrochloric, hydrofluoric and phosphoric acids and their salts; organic substances such as hydrocarbons, fertilizers, plant protection and pest destruction agents, plastics, man-made fibres, paints and lacquers, pharmaceutical products, adhesives, detergents, cosmetic products, polishing powders, and leather and textile accessories.
The principal emissions given off by the chemical industry are gases and vapours of organic chemical compounds such as hydrocarbons and their halogen derivatives, aldehydes, ketones, carboxylic acids, and nitrogen and sulphur compounds (amines, mercaptans, sulphides); gases and vapours of inorganic chemical compounds such as hydrogen sulphide, hydrochloric acid, fluorine compounds, sulphur dioxide, and hydrogen phosphides; and finally toxic powders such as fluorides and carbides and powders from iron alloys, arsenic and asbestos.
Extensive control is necessary, partly because the human sense of smell is very sensitive to substances emitted by chemical industries (such as hydrogen sulphide and ercaptans), even when they are highly rarefied.
To eliminate these substances, purification plants of very high degree of efficiency are required. Furthermore, the chemical reactions take place in many cases under very heavy pressure.
For safety reasons the reaction apparatuses and accessories must therefore, be equipped with pressure relief facilities (safety valves). In the event of breakdowns, which can be fairly frequent in a chemical works, the noxious substances are released in relatively large quantities.
d. Petroleum Industry:
The petroleum industry is characterized by the regular discharge of hydrocarbons and other organic compounds and of sulphur dioxide and fine dust. Crude oil imported form the oil-producing countries is processed in refineries into various petroleum products, in particular heating oil and gasoline.
In the refining process, the crude oil is heated and vaporized and then condensed again at various temperatures. In this way the varying fractions, from highly volatile gasoline to heavy heating oil, are separated. In the process, tarlike bitumen is left behind for further processing in its turn.
In order to produce the energy needed to vaporize the crude oil, the distillation residues are used in the refineries as the main fuel. These residues contain large quantities of sulphur, which after combustion, is given off into the atmosphere in the form of sulphur dioxide. In addition, according to where it comes from, the material consumed can contain larger or smaller quantities of fine dust, which is also given off with the flue gas.
The second group of components in air pollution by refineries are organic substances such as hydrocarbons. These are discharged in the main from various sources, at ground level, spread over the entire area of the works, such as storage tank, flanges, safety valves, vents, seals, settling tanks and torch flarges. These organic gases and fumes are in some cases very pungent, and so can cause serious nuisance in the vicinity of the refinery.
It is assumed at present that one-third of production losses by the petroleum industry pass into the atmosphere as hydrocarbons. Discharges into the environment also occur when finished products are loaded into vehicles and vessels for distribution.
Each time gasoline is loaded into a container or tanker for transport by land or sea, gas vapour will be discharged into the atmosphere with the expelled air to the amount of 1.3% of the gasoline transported.
The situation is very similar in the case of petro-chemistry. Petrochemical production plants are usually situated in close proximity to refineries, and in them the end products from the refineries are processed further.
The petrochemical plants also contain extensive pipeline systems and numerous valves and flanges through which hydrocarbons and other organic compounds are discharged. It is estimated that on an average some 1% of the substances processed in petro-chemistry escape in the production process.
e. Pollution form Spray Gun:
A good example of serious irreversible damages to the environment caused by an industrial product which was previously regarded as neutral in its effects is the spray gun, which is in increasing use today for a variety of purposes.
As a propellant, the spray gun contains chlorofluorocarbons (CF2CL2and CFCL2). When the spray gun is used, the propellants are released into the atmosphere. Until recently it was believed that the compounds were so stable that they would not be absorbed or reduced by either plants, soil or water.
They were consequently regarded as harmless. In 1974, however, American scientists at the University of Michigan discovered that chlorofluorocarbons could cause serious disturbances in the environment.
The gas rises into the stratosphere and damages the ozone belt surrounding the earth; the ozone belt intercepts a large part of the ionizing radiation coming in from outside and so reduces damage to the earth by this radiation.
f. Industry & Power Generation:
The two major air pollutants produced by industry and power stations are sulphur dioxide and nitrogen oxides. Sulphur dioxide is the main pollutant emitted from power stations. In Britain, 90% of sulphur dioxide pollution comes from power stations and industry.
Once in the air, sulphur dioxide mixes with moisture to form sulphuric acid, which will later fall as acid rain. Nitrogen oxides are pollutants which are mainly associated with car exhausts, although some come from industry. Again, they mix with moisture in the atmosphere to form nitric acid, which then falls as acid rain.
Today, industries that emit air pollution must have chimneys or stacks which release the waste gases into the atmosphere at higher levels, thereby reducing the risk of ground level pollution. Power stations are now also required to reduce the amount of sulphur dioxide they give off by using specialised clean technology.
Essay # 3. Sources of Air Pollution:
Air pollution is the result of the combined effects of several pollutants. These pollutants are associated with each other and also react with other elements, therefore, it is difficult to categories them, but for proper understanding they can be divided into the following categories on the basis of their origin, nature, size, impact, etc.
1. According to Origin, particulate matter can be divided into two types, viz., natural and man-made. The natural form of particulate matter is the result of volcanic dust and gases, mineral dust, sea-salt crystals.
Another class of natural particulate matter is smoke from forest fires and grass fires. Living plants release pollens and spores into the air, these are organic compounds. From forest trees certain hydrocarbons called terpenes are also released into the atmosphere.
Man-made particulate matter comes from many sources, but the major source is from the combustion of hydrocarbon fuels—petroleum products, coal, peat and wood. Combustion of solid wastes is another source.
Other kinds of matter introduced into the atmosphere are industrial chemicals, fly ash, refining fossil fuels, mining and smelting ores, as well as pollutants discharged through quarrying, farming activities, etc. Use of various types of solvents and also radio-active elements are the cause of air pollution created by man.
2. Another classification, according to Origin, is (i) primary, and (ii) secondary pollutants. The primary pollutants are those gaseous and other solid micro particles inducted into the atmosphere. These pollutants are emitted and as such are not found in the air.
The most important gaseous pollutants are carbon monoxide, oxides of sulphur, hydrogen sulphide, hydrocarbons, oxides of nitrogen, ozone and other oxidants. Secondary pollutants are the result of chemical reactions. Evidences and experiments indicate that exhaust gases of automobiles contribute more in the formation of secondary pollutants.
For example, oxides of nitrogen produced in the combustion of petroleum and other fuels emitted into the atmosphere, yield ozone in the presence of sunlight. It is to be noted that ozone is not emitted as such into the atmosphere but formed only from primary pollutants.
3. According to chemical composition air pollutants can be divided into organic pollutants and inorganic pollutants. Others have divided them into solid, liquid and gaseous pollutants.
The gaseous pollutants are carbon monoxide, sulphur dioxide, hydrogen sulphide and organic sulphide, hydrogen fluoride, hydrogen chloride, oxides of nitrogen, aldehydes and organic acids, etc. Particulate pollutions consist of both solid and liquid particles. They vary in size from 0.01 micron to 20 microns. Dust, fume, mist, spray, smoke are included in this category.
4. According to source type, pollutants can be classified as being produced from: (i) Combustion, (ii) Transportation emissions, (iii) Industrial process, (iv) Use of solvents and (v) Radioactivity.
Combustion process yield particulates such as fly ash and smoke and oxides of sulphur and nitrogen. The amount of sulphur dioxide emitted depends upon the contents in the fuel. High temperature processes such as thermal fixation of atmospheric nitrogen yield larger quantities of oxides of nitrogen. Carbon monoxide is also emitted from combustion. The other contaminants that are produced by combustion include acids and aldehydes.
The simplest form of combustion is the use of fuels in domestic use. In India as well as in many developing countries, wood, coal, cow dung and kerosene oil are commonly used. All these materials yield carbon dioxide, carbon monoxide, sulphur dioxide, etc.
Automobiles may be considered as the main source of air pollution, specially in urban areas.
The rapid rate of industrialisation has resulted in more and more air pollution.
Nuclear material, when released into the air, is hazardous for all living organisms. Nuclear weapon testing’s, nuclear reactors, chemical processing plants, research institutes and hospitals contribute many radionuclides to the atmosphere.
An average adult exchanges about 15 kg of air every day. Pure air is a mixture of gases (78% nitrogen, 20.95% oxygen, 0.03% carbon dioxide, 0.93% inert gases, 0.00005% hydrogen and variable amount of water vapour). Air is never found clean in nature due to natural and human activities.
The concentration level of the above gases show considerable variations along with the presence of a number of harmful gases and particles of solid or liquid due to pollution. The pollutants causing air pollution hardly exist beyond 2,000 feet (600 m) above the ground level.
Air pollution is the presence of gases and other substances in concentrations that disturb the dynamic equilibrium of the atmosphere and cause hazards to human health harm to living resources and ecological systems, damage to structures or amenity or interference with legitimate use of the environment.
Air pollution is one of the most dangerous and common kind of environmental pollution that is reported in most industrial towns and metropolitan cities of India and abroad. Incidences of air pollution are numerous and contributors to air pollution have increased over the decade.
As air is mobile, one cities problem yesterday has become a concern of another city today. Urban areas too have spread so rapidly that industries once outside the city boundaries are today well within the city limits.
Sampling of air pollutants are generally done by thermal and by electrostatic precipitation, Sonkin impactor and electrostatic dust collectors. Particulate pollutants are measured by deposit gauge or by Owen’s dust counter, Liegean sphere and Ringel-mann chart are used to measure the thickness of smoke.
Some gases like fluoride are estimated by colour reactions. Estimates of SO2 in air is made by chemical analysis of the dust collected in a deposit gauge or by a bubbler method.
Air pollution sources are natural and due to the activities of mankind:
A. Natural sources:
Air pollution from natural sources include volcanic eruptions, naturally occurring forest fires, airborne pollen dust from deserts and storms, dust from erosion and gases produced by decay. Volcanic eruptions lead to the injection of large quantities of gases and particulate matter into the atmosphere. Particles of volcanic ashes may penetrate the stratosphere, spread widely and may lead to climactic cooling.
About 57% of sulphur dioxide (SO2) produced globally from hydrogen sulphide gas, comes from natural sources. Forest fires contribute about 7.2% of carbon monoxide (CO) and 5.8% of nitrogen oxide (NO) emissions. Natural sources of hydrocarbons come from forest fires and bacterial decomposition of organic matter. Pollen grains are often carried by dust and storm which may affect the people sensitivity to it.
B. Anthropogenic sources:
Anthropogenic sources of air pollution are the result of human activities which are thought to be as old as our ability to start fire. Man made sources of pollution are due to industries, automobiles, thermal power stations etc. Their effects may be small on a global scale but are very severe locally. Anthropogenic sources of pollutants can be classified as stationary combustion sources, mobile combustion sources and manufacturing sources.
(a) Stationary combustion sources:
Stationary combustion sources include the use of coal or petroleum and the principal emission are particulate pollutants like fly ash and smoke, oxides of sulphur and nitrogen. Incomplete combustion of carbons and hydrocarbons produces carbon monoxide.
Among the worst stationary sources are the power plants. The fly ash emitted from this plant reduces visibility and contains traces of arsenic, cadmium, lead, manganese, beryllium and fluorine. Another important stationary source is the burning of biomass fuel and coal for domestic cooking and room heating at high altitudes.
Emission from this source includes carbon monoxide, oxides of sulphur and nitrogen, aromatic hydrocarbons, formaldehyde and particulate matters. Women and children are affected most from exposures to such burning of biofuels. About 200 million women in developing countries are exposed to such conditions which is similar to smoking 2-20 packs of cigarettes per day.
(b) Mobile combustion sources:
Mobile combustion sources are from gasoline, diesel fuel and jet fuel used for mobile engines. As these are hydrocarbons, the pollutants released when they are burnt are similar to those from stationary combustion sources.
(c) Manufacturing sources:
Manufacturing sources are the materials that are used in industries, commerce and house holds, resulting in the release of unwanted particulate matters and gasses into the atmosphere.
The sources of air pollution can also be divided into:
(a) Industrial sources:
Industries are potent sources of air pollution. The major culprit being the petroleum refineries that are major sources of gaseous pollutants like SO2, NOx etc. About one-fifth of the air pollution comes from industrial processors such as metallurgical plants and smelters, pulp and paper mills, sugar mills, cotton mills and synthetic rubber manufacturing plants.
Cement factories and stone crushers create plenty of dust. The suspended particulate matter (SPM) are above the industrial safely limits in these areas and cause health hazards. Acid vapours are emitted into the air by chemical industries.
(b) Automobile sources:
Automobiles such as scooters, cars, trucks, buses, air- crafts, ships, rails etc. contribute substantial amount of pollutants into the air. The ever-increasing vehicular traffic density possess continuous threat to the surrounding air quality.
The sources of pollutant emission from automobiles are the:
(i) Exhaust system,
(ii) Fuel tank and carburettor
(iii) Crank case.
The exhaust produces many pollutants such as un-burnt hydrocarbons, CO, NOx and lead oxides and traces of aldehydes, esters, ethers, peroxides and ketones. As fuel tanks contain petrol which volatile in nature, it results in the emission of hydrocarbon into the air.
Such emission of hydrocarbons also occur due to evaporation through carburetor when the engine is stopped and heat builds up. The crank case also discharges hydrocarbon into the atmosphere.
(c) Thermal power sources:
The chief pollutants of thermal power station due to the burning of coal are fly ash, SO2, NO2, CO, aldehydes and hydrocarbons.
(d) Other sources:
Other sources of air pollution are of minor consequences, but they release some harmful tonic substances. Agricultural practices release pesticides and dust into the atmosphere. Forest and field burnings release CO and NOx.
Essay # 4. Effects of Air Pollution:
Air pollution has now become a widespread problem and every individual in one way or other is facing problems caused by air pollution.
The effects of air pollution can be grouped under the following heads:
a. Effects on Human Health:
Some environmental poisons can cause acute illness and even death. Others may be harmful, but the disease may take years or even decades to appear. Air pollution mainly affects the respiratory system. Bronchitis, emphysema, asthma and lung cancer are some of the chronic diseases caused due to exposure to polluted air.
It is feared that lung cancer is caused mainly due to polluted air because carcinogens are found in the polluted air. Sulphur dioxide is the most serious and widespread air pollutant. Its low concentration is a cause of spasms in the smooth muscle of bronchioles and its higher concentration induces increased mucus production.
Sulphur dioxide is also considered to cause cough, shortness of breath, spasm of the larynx and acute irritation to the membranes of the eyes. So, also acts as an allergenic agent. When it reacts with some compounds, sulphur acid is formed which may damage lungs.
Carbon monoxide often affects the oxygen carrying capacity of blood. Nitric oxide is reported to be a pulmonary irritant and its excess concentration may cause pulmonary haemorrhage. Hydrogen sulphide is also toxic. Lead emitted from automobile exhausts is a cumulative poison and is dangerous particularly to children and may cause brain damage.
The particulate pollutants such as asbestos, silica, carbon, beryllium, lead, etc., are capable of exerting a noxious (fibrotic) local action in the interstitial areas of the lungs. Radio-active elements are also harmful to man and other living organisms. In fact, the growing air pollution has now become a health hazard for man.
b. Effect on Animals and Plants:
The impact of air pollution on animals is more or less similar to that of effects on man. Chronic poisoning results from the ingestion of forage contaminated with atmospheric pollutants. Among the metallic contaminants arsenic, lead and molybdenum are important.
Fluoride is another pollutant which causes fluorosis among animals. A number of livestock have been poisoned by fluorides and arsenic in North America. Bone lesions in animals due to excessive fluorides have also been reported.
Air pollution has caused widespread damage to trees, fruits, vegetables, flowers and in general, vegetation as a whole. The total annual cost of plant damage caused by air pollution in USA alone has been estimated to be in the range of 1 to 2 billion dollars.
The most dramatic early instances of plant damage were seen in the total destruction of vegetation by sulphur dioxide in the areas surrounding smelters. When the absorption of sulphur dioxide exceeds a particular level, the cells become inactive and are killed, resulting in tissue collapse and drying of leaves. Cotton, wheat, barley and apple are more sensitive to this pollutant.
Fluorides are responsible for various types of injuries to plants. The leaves of apple, apricot, peach, prune are more susceptible to air borne fluorides. Fluorides are seen to interfere with the photosynthesis and respiration of plants. Smog also causes injury to plants. Similar impact of ozone can be seen in the lesions to plants. Chlorine, ammonia, hydrogen sulphide, etc., are also harmful to vegetation.
i. Acid rain (as a by-product of atmospheric pollution) may acidify lakes and streams and kill fish and aquatic plants.
ii. Pollution may affect animals through plants on which they feed.
For example, if a certain plant is negatively affected by air pollutants, this will also affect the animals that depend on this particular plant for food.
Sulphur dioxide and nitrogen dioxide are transformed in the atmosphere to produce acid compounds-sulphuric and nitric acids. These compounds then fall back on to the ground as particulates or raindrops-in other words, acid rain.
Acid rain falls on streams and lakes, acidifies them and destroys fish life in freshwater ecosystems.
For example, in Sweden acid rain made over 18,000 lakes so acidic that all the fish died.
Some other populations of animals in Europe and North America have also been declining due to acid rain.
i. Pollution may also affect animals through plants on which they feed.
For example, pea aphids feed on pea plants exposed to sulphur dioxide in the air. High exposure to sulphur dioxide negatively affects the health of the pea plants, and therefore, the health of the aphids as well.
Some other examples of effects on animals of air pollution are:
i. Excessive ultraviolet radiation coming from the sun through the ozone layer in the upper atmosphere which is eroded by some air pollutants may cause skin cancer in wildlife.
ii. Tropospheric ozone may damage lung tissues of animals.
It is also probably logical to assume that many higher order animals (especially those closely related to humans, e.g., mountain gorillas) experience air pollution effects similar to those experienced by human beings.
c. Effects of Air Pollution on Forests, Trees & Plants:
Air pollution can have both long-term and short-term effects on plants.
i. Physical injury to leaves is the immediate effect of air pollution on plants which are generated due to.
ii. Ozone produces a speckle of brown spots, which appear on the flat areas of leaves between the veins.
iii. Sulphur dioxide: larger bleached-looking areas.
iv. Nitrogen dioxide: Irregular brown or white collapsed lesions on intercostals tissue and near the leaf edge.
v. Ammonia: unnatural green appearance with tissue drying out.
vi. Of all main air pollutants, sulphur dioxide often comes up as the one that most negatively affects plants & trees.
Lichens are considered to be most sensitive to sulphur dioxide. During the period of high levels of sulphur pollution, large parts of Europe lost many species of lichen and became known as “lichen deserts”.
Sulphur dioxide may also affect higher plants, including wild species, crops and trees (through) some species may develop sulphur dioxide tolerant populations in response to long-term to long-term exposure).
These effects may be:
i. Cell metabolism disruption (membrane damage, respiration and photosynthetic effects).
ii. Leaf injury and loss.
iii. Reduced growth and reproduction.
iv. Increase in susceptibility of plants to attacks by insect herbivores.
v. Nitrogen dioxide, another air pollutant, may act in synergy with sulfur dioxide to produce a negative effect on plants’ photosynthesis.
vi. Troposphere ozone can prevent plant respiration by blocking stomata and negatively affecting plants’ photosynthesis rates which will stunt plant growth; ozone can also decay plant cells directly by entering stomata.
vii. Particles, just like ozone, often affect plants & trees via blocking of leaf stomata through which plants undertake the gas exchange necessary for photosynthesis and respiration.
Dust particle form a smothering layer on leaves, reducing light and hence lowering photosynthesis rates.
viii. Many dust are inert and so only act by shading.
ix. However, some dusts are also chemically active.
x. Thus cement dust will also dissolve leaf tissue, resulting in additional injury.
xi. Coal dust may also contain toxic compounds.
Dusts may also affect ecosystems through their action on soil. Thus the alkaline chemistry of limestone dusts can raise the soil pH of acid and neutral habitats, resulting it the loss of plant and animal species.
Acid rain (a product of air pollution) severely affects trees and plants as well. It can kill trees, destroy the leaves of plants; can infiltrate soil by making it unsuitable for purpose of nutrition and habitation.
It is also associated with the reduction in forest and agriculture yield.
d. Smog Formation:
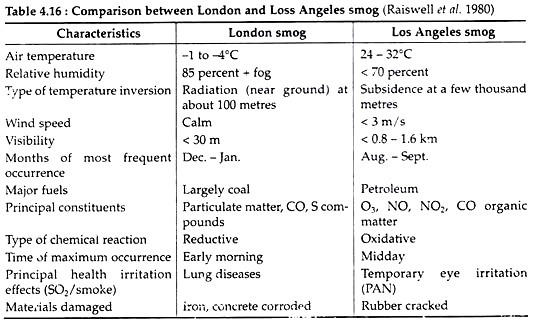
One of the most hazardous effects of air pollution is the classical smog. The word smog is coined by combining smoke and fog which was associated with London, Glasgow, Manchester and other cities of U.K., where sulphur-rich coal was used. The term was coined by H. A. Des Voeux in 1905. The UK smog was a mixture of reducing pollutants and has been termed as reducing smog.
On the contrary, photochemical smog is much more complex and was first reported from Los Angles in 1943. It is also termed oxidising smog as it contains a mixture of oxidising pollutants. The comparison between Los Angeles and London smog is given in Table 4.16.
The pollutants that are responsible for the Los Angeles smog are not emitted directly from any source. These are the secondary pollutants, formed in the atmosphere as a result of chemical reactions involving the primary pollutant/ Nitrogen dioxide in the atmosphere dissociates when irradiated by intense, short-wavelength, radiant energy from the sun and a series of photochemical reactions lead to the formation of the powerful oxidant, ozone (O3) in the following way:
where h represents an input of short-wavelength radiation and M represents a molecule that acts somewhat as a catalyst. This M may react with O and O3 to form new compounds mainly of solid particles. Some reactive hydrocarbon (HC) species or volatile organic compounds (VOC’s) are effective in this role.
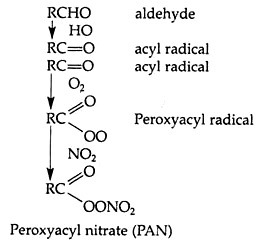
Peroxyacetyl nitrate (PAN) is another secondary pollutant formed which has the ability to cause severe plant damages even at low concentrations.
The probable mechanism for PAN formation is:
Photochemical smog is very much prevalent in big cities of India like Kolkata, Mumbai, Delhi, Chennai, Bangalore, Kanpur etc. The chief sources of smoke in these cities are automobiles and industries. Mumbai, in 1987, experienced a smog for about 10 days.
Photochemical smog adversely affects human health, plants and materials. Serious outbreaks of smog cause asthma and bronchitis in epidemic form. Tokyo-Yokohama asthma occurred in Yokohama, Japan in 1946. Another serious disease is emphysema which results in structural breakdown of the alveoli of lungs.
PAN is known to block “Hill reaction” of photosynthesis and results in bronzing and glazing of abaxial leaf surface. It is due to plasmolysis and collapse of mesophyll cells. Smoke and particulate matters in smog reduce the visibility, damage crop and livestock and cause corrosion of metals, stones, building materials, textile, paper leather, painted surface etc.
e. Effects on Atmosphere:
There is an increase in the carbon dioxide concentration in the air due to increased combustion of fossil fuels. Carbon dioxide absorbs heat strongly and the radioactive cooling effect of the earth is thus decreased. The rising of temperatures and ozone holes are some of the problems which have attracted the attention of the scientists of the world.
These problems are not related to any region or a country but are the global problems and their impact on world climate may be hazardous to the whole world. The local weather conditions are highly susceptible to air pollution. Its impact on temperature, humidity, rainfall and clouds is apparent. The ‘smog dom’ on large urban centres are the result of air pollution. Due to air pollution visibility also reduces.
f. Effects of Air Pollution on Environment:
Acid rain and green-house effect are the major effects of air pollution on environment.
The have adverse effects on:
i. Forests & other vegetation
ii. Freshwater lakes & streams destroying aquatic life
iii. Soil and
iv. Buildings & materials
g. Green House Effect:
The temperature at the surface of the earth is maintained by the energy balance between the sun’s rays that strike the planet and the heat that is radiated back into space. Some of the heat is absorbed and retained by the earth or objects on the surface.
Much of this does not pass through the air envelope to outer space but is absorbed by the carbon dioxide and water vapour in the atmosphere and adds to the heat that is already present.
Thus carbon dioxide acts like the glass of a greenhouse, and on a global scale, tends to warm the air in the low levels of the atmosphere. This is called the greenhouse effect which is also responsible for the increase in temperature over the earth’s surface.
Scientists are of the opinion that carbon dioxide will increase global temperature significantly. There is no two opinion about the fact that due to industrial and other forms of air pollution the carbon dioxide content is increasing.
Volcanic eruptions are also responsible for the increase of carbon dioxide. Volcanic activity during historical period as well as in present times has thrown out dust and ash which spread throughout the entire atmosphere and last for years.
With the increase in temperature, scientists have predicted that the process of melting of ice caps of Antarctic, Greenland, etc., may lead to a rise in the sea level. Some of them have pointed out that there is a rise of four indies in every ten years.
There is a crucial relationship between the presence of the polar ice cap and global temperatures and if it happens its impact on climatic conditions of the world will be drastic and change the entire pattern of our ecosystem.
h. Economic Losses Due to Air Pollution:
Apart from direct health related and other environment issues, air pollution brings with it economic losses as well.
Some of the economic losses caused by air pollution are as follows:
i. Direct medical losses
ii. Lost income from being absent from work
iii. Decreased productivity
iv. Travel time losses due to reduced visibility
v. Losses from repair of damage to buildings
vi. Increased cost of cleaning
vii. Losses due to damage to crops & plants and
viii. Losses to historical buildings, e.g. Taj Mahal in Agra.
We can only imagine the economic losses suffered by developing countries such as China, where air pollution level is among the highest in the world.
i. Other Effects:
Air pollution can also cause damage to property and materials. The smoke, grit, dust and oxides of sulphur have harmful effects on structures. In 1972, when an oil refinery at Mathura was opened, its impact on Taj Mahal became a major issue. Sulphur dioxide is the most damaging of gaseous pollutants.
Aluminium alloys, copper and copper alloys, iron and steel are corroded when exposed to contaminated air. Hydrogen sulphide reacts with lead paints to form lead sulphide thereby producing a brown to black discolouration. The damage caused by air pollution to structures is not serious but from an aesthetic point of view it is not desirable.
Essay # 5. Consequence of Air Pollution:
Some of the major consequence of air pollution are as follows:
a. Rainfall Acidity:
Rainfall is naturally acidic due to the presence of carbon dioxide in the atmosphere which combines with rainwater to from weak carbonic acid. However, the combustion of fossil fuels produces waste gases such as sulphur dioxide, oxides of nitrogen and to a lesser extent, chloride.
These pollutants can be converted, through a series of complex chemical reactions, into sulphuric acid, nitric acid or hydrochloric acid, increasing the acidity of the rain or other type of precipitation, such as snow and hail.
Carbon dioxide + water = Carbonic acid (weak)
Sulphur dioxide + water = Sulphuric acid
Nitrogen oxides + water = Nitric acid
Acid emissions of sulphur dioxide and nitrogen oxides arise from many industrial sources as a result of combustion processes. In the UK power stations contributed 65% of all sulphur dioxide emitted in the UK in 1999. Other industries were responsible for 22%.
Industries also emit nitrogen oxides which can also cause rainfall to become more acidic. While road transport is the major source of nitrogen oxides in the UK (44% in 1999), power stations accounted for 21% and other industries 13% in 1999. There are many technologies which can be used in industry to reduce the emissions of pollutants to the atmosphere and these can be applied before, during or after combustion.
Examples of pre-combustion sulphur control technology (removing sulphur before burning) include coal scrubbing and oil desulphurisation. Another removal process is to change the design on the boiler and to install pressurised fluidized bed combusters (FBC) which removes sulphur from coal during combustion is the Integrated Gasification Combined Cycle.
Coal is gasified under pressure with a mixture of air and steam which results in the formation of gas which can then be burned to produce electricity.
One of the post-combustion sulphur controls (removing sulphur after burning) is Flue Gas Desulphurisation (FGD). In FGD processes, waste gases are scrubbed with a chemical absorbent such as limestone to remove sulphur dioxide. There are many different FGD processes, the main ones being the limestone-gypsum process and the Wellman- Lord regenerative process.
The limestone-gypsum FGD involves mixing limestone and water with the flue gases to produce a slurry which absorbs the sulphur dioxide. The slurry is then oxidised to calcium sulphate (gypsum) which can then be used in the building trade.
Unlike sulphur, it is not possible to reduce the nitrogen content of the fuel before combustion of the fuel by physical cleaning as it is combined with the organic matter of the fuel, and at present there are no commercially available methods to reduce organic nitrogen.
Instead, nitrogen oxides can be removed during combustion. Low nitrogen oxides burners ensure that the fuel is burnt in low oxygen concentrations, such that any nitrogen oxides produced are reduced to nitrogen gas. Once initial combustion has taken place, further air is added to the combustion chamber to ensure that the fuel is completely burnt.
Advanced low nitrogen oxides burners can reduce emissions by up to 30%. Such burners can be installed on either new or existing combustion plants.
Emissions of nitrogen oxides, like for sulphur dioxide, can also be reduced by treating the flue gases. One method involves mixing the flue gases with ammonia, converting the nitrogen oxides to nitrogen and water. This process is suitable for fitting to existing plants and new build applications, and can achieve an emissions reduction of up to 80 to 90%.
Some fuels, for example natural gas, are naturally less polluting in terms of acidic emissions, whilst traditional coal power generation is more polluting, depending on the amount of sulphur there is in the coal being burnt. To help reduce atmospheric emissions of sulphur dioxide and nitrogen oxides in the UK, many of the more recent power stations have been built to operate on gas rather than coal.
b. Acid Deposition:
Acid rain is a general name for many phenomena including acid fog, acid sleet and acid snow. Although we associate the acid thread with rainy days, acid deposition occurs all the time, even on sunny days. Acid deposition is the scientific term used to describe “Acid Rain”.
When atmospheric pollutants such as sulphur dioxide and nitrogen oxides mix with water vapour in the air, they are converted to sulphuric and nitric acids. These acids make the rain acidic, hence the term “acid rain”. Rain returns the sulphur and nitrogen acids to Earth, and in high concentrations, can cause damage to natural environments including forest and freshwater lakes.
This form of acid deposition is known as wet deposition. A second method of acid deposition is known as dry deposition. Whilst wet deposition involves the precipitation of acids, dry deposition occurs when the acids are first transformed chemically into gases and salts, before falling under the influence of gravity back to Earth. Sulphur dioxide, for example, is deposited as a gas and as a salt.
The gases present in the acid deposition are found to occur naturally in the environment. They are given off from a number of sources including volcanic eruptions and the rotting of vegetation’s. They become a problem when humans produce the gases in large amounts, and at high concentrations by the burning of fossil fuels.
The distances that pollutant gases travel means that acid deposition is an international or trans-boundary problem. This means that acid pollutants are not necessarily deposited in the same country’ where they were produced.
c. Acidic Emissions:
Rain water is naturally acidic as a result of carbon dioxide dissolved in water and from volcanic emissions of sulphur. However, it is the chemical conversion of sulphur and nitrogen emissions from power stations, factories, vehicles and homes, where fossil fuels are burnt, that we call acid rain. These waste gases are carried by the wind, sometimes over long distances, and can in time be converted into sulphuric and nitric acids.
Natural sources of sulphur dioxide (SO2) include releases from volcanoes, oceans, biological decay and forest fires. Actual amounts released from natural sources in the world are difficult to quantify; in 1983 the United Nations Environment Programme estimated a figure of between 80 million and 288 million tonnes of sulphur oxides per year.
Man made sulphur dioxide emissions result from combustion or burning of fossil fuels, due to varying amounts of sulphur being present in these fuels. Worldwide emissions of SO, are thought to be around 69 million tonnes per year.
Levels of sulphur dioxide from combustion sources in the UK have declined in recent decades. Between 1970 and 1999, UK sulphur dioxide emissions fell by 82% due to recession, restructuring of industry, substitution of fuels (for example natural gas) and air pollution control technology.
Power station emissions fell by 73% over the same period, but the percentage of UK emissions from power stations has actually increased to 65% of the 1999 total compared to 45% of the total in 1970.
Natural sources of nitrogen oxides (NOx) include volcanoes, lightening strikes and biological decay. Estimates range from between 200 million and 90 million tonnes per year NOx from natural sources, compared to around 24 million from human sources world-wide. Nitrogen oxides are produced when fossil fuels are burned.
The major sources of NOx in the UK in 1999 were road transport (44%), power stations (21%) and industry (including iron and steel, and refineries) (12%). Emissions of nitrogen oxides from road transport increased during the 1970s and 1980s before decreasing again during the 1990s.
For example, in 1970, emissions of NOx from road transport in the UK were 0.769 million tonnes by 1990 they had risen to over 1.31 million tonnes NOx. Since then, however, emissions from transport have been declining due to improvements in vehicle technology, such as the use of catalytic converters, and the use of cleaner fuels. In 1999 they were 0.714 million tonnes, lower than in 1970.
The geographical distribution of human acidic emission sources is not even. Nitrogen and sulphur emission sources are heavily concentrated in the Northern Hemisphere, particularly in Europe and North America. As a result, precipitation is generally more acidic in these countries, with an acidity in the range of pH 4.1 to pH 5.1. ‘Normal’ or ‘unpolluted’ rainfall has a pH of 5.6.
d. Critical Loads:
Critical loads have been defined as: “the highest load that will not cause chemical changes leading to long-term harmful effects in the most sensitive ecological systems”. Critical loads are the maximum amount of pollutants that ecosystems can tolerate without being damaged. The definition has been redrafted in order to fit specialist areas of interest, most particularly the acidification of freshwater and soils.
The concept behind critical loads is upon a dose-response relationship where the threshold of harmful response (within the ecosystem) is triggered by a certain load of pollutant—the critical load. However, it is not always easy to apply without careful consideration of the nature of the affected ecosystem and the threshold effects of harmful pollutants.
In order for critical loads to be used, target loads need to be set for different areas in order to try and halt the acidification processes. Target loads have been defined as “the permitted pollutant loads determined by political agreement”.
Therefore, target loads can be either higher or lower than the critical load values. For example, the target loads may be lower so as to give a safety margin or the target loads may be higher for economic reasons.
The reasoning behind this is that critical loads only show where there is an acidification problem and to what degree damage is occurring. Target loads are used in order that emissions can be reduced accordingly to meet the targets and limit the amount of damage.
The critical loads for total acidity of sulphur and nitrogen need to be determined so that a coherent international agreement can be reached with regard to abatement policies. There are numerous methods that are available for obtaining critical loads.
In order to obtain values for the critical loads, an ecosystem has to be chosen and then a suitable indicator species is selected to represent the ecosystem. A chemical limit is subsequently defined as the concentration at which the indicator species will die. In forests the indicators are trees, and in freshwaters they are fish.
Through the Convention on Long-Range Trans boundary Pollution, member countries of the United Nations Economic Commission for Europe (UNECE) agreed that the critical loads approach provided an effective scientific approach for devising strategies for the abatement of acid deposition.
The UK Government accepted that the critical loads approach was the best way to establish abatement strategies in relation to sulphur dioxide and nitrogen oxides emissions. It was recognised that critical loading maps were essential in providing information on the geographical distribution of pollutant- sensitive locations.
The Critical Loads Advisory Group (CLAG) was set up to produce critical load maps for the United Kingdom. The National Centre for Critical Loads Mapping was subsequently established at the Institute of Terrestrial Ecology, now the Centre for Ecology and Hydrology.
Critical load maps of soil acidity have been produced for the UK at a grid resolution of 1 km squares. Maps for the critical acidity of freshwater environments are based on a single water sample from a single site in each of the 10 km squares used, assumed to be the most sensitive surface water within the grid square.
These critical loads maps, when combined with acid deposition values, produce exceedance maps which show where and by how much the critical loads are being exceeded. Maps are available for soils in the UK relating to acidity and sulphur deposition showing areas that are sensitive to acidification.
These correspond to areas where have been reports of acidification. In the UK a national target load map for the year 2005 has been produced for soils on a 20 km by 20 km grid system, showing the target loads that need to be met for such areas.
In 1997, critical loads for acidification were exceeded in 71% of UK ecosystems. As sulphur deposition continues to fall, this value is expected to fall to below half by 2010, when nitrogen deposition will dominate.
Critical loads for eutrophication (nutrient depletion) in 1997 were exceeded in about a quarter of UK 1 km by 1 km squares with sensitive grasslands and a little over half with heathland. Again, this is expected to decline over the next 10 to 15 years.
As resulting in less air pollution there are many ways to help reduce air pollution which causes acid rain. Use buses and trains instead of cars, as they can carry far more people in one journey. This cuts down the amount of pollution produced. Walking or cycling whenever you can will by even more beneficial, as it does not create any pollution. It will also benefit your body, as regular exercise will keep you fit and healthy.
If your parents must use the car, ask them to avoid using it for very short journeys if possible, as this creates unnecessary pollution. Try to encourage them to share their journeys with other people, for example, when they go to work or go for shopping. Also encourage them to drive more slowly as this produces less pollution.
We can also help prevent pollution from our own homes which may contribute to acid rain. Turning off lights when they are not needed and not wasting electricity will reduce the demand. Less electricity will need to be produced and so less coal, oil and gas will have to be burnt in power stations pollution.
Essay # 6. Air Pollution in India:
Industrialization and urbanization have resulted in a profound deterioration of India’s air quality. Of the 3 million premature deaths in the world that occur each year due to outdoor and indoor air pollution, the highest number are assessed to occur in India. According to the World Health Organisation, the capital city of New Delhi is one of the top ten most polluted cities in the world.
Surveys indicate that in New Delhi the incidence of respiratory diseases due to air pollution is about 12 times the national average. According to another study, while India’s gross domestic product has increased 2.5 times over the past two decades, vehicular pollution has increased eight times, while pollution from industries had quadrupled.
Sources of air pollution, India’s most severe environmental problem, come in several forms, including vehicular emissions and untreated industrial smoke. Apart from rapid industrialization, urbanization has resulted in the emergence of industrial centres without a corresponding growth in civic amenities and pollution control mechanisms.
Regulatory reforms aimed at improving the air pollution problem in cities such as New Delhi have been quite difficult to implement, however. For example, India’s Supreme Court recently lifted a ruling that it imposed two years ago which required all public transport vehicles in New Delhi to switch to compressed natural gas (CNG) engines by April 1,2001.
This ruling, however, lead-to the disappearance of some 15,000 taxis and 10,000 buses from the city, creating public protests, riots, and widespread “commuter chaos”. The court was similarly unsuccessful last year, when it attempted to ban all public vehicles that were more than 15 years old and ordered the introduction of unleaded gasoline and CNG.
India’s high concentration of pollution is not due to lack of effort in building a sound environmental legal regime, but rather to a lack of enforcement at the local level.
Efforts are currently underway to change this as new specifications are being adopted for auto emissions, which currently account for approximately 70% of air pollution. In the absence of coordinated government efforts, including stricter enforcement, this figure is likely to rise in the coming years due to the sheer increase in vehicle ownership.
Air pollution is considered to be one of the most dangerous and common kinds of environmental pollution that has been reported in most industrial towns and metropolitans of India and abroad, such as Delhi, Bombay, Calcutta, Kanpur, Chennai, Hyderabad, Jaipur, Ahmedabad, Nagpur, Firozabad, Tuticorin etc. In foreign countries, London, New York, Tokyo and Pittsburg etc., were worst affected by air pollution.
The National Environmental Engineering Research Institute (NEERI), Nagpur initiated the air quality monitoring programme in 1978 in 10 cities in India. It provides data to study the air quality trends on a long-term basis.
The analysis of data of NEERI revealed that annual mean values of the SPM indicated positive trends in Bombay, Cochin and Jaipur, S02 levels also showed a positive trend in Bombay, Cochin and Nagpur, NO2 levels showed a positive trend in Bombay, Calcutta, Cochin, Delhi, Hyderabad, Kanpur, and Nagpur.
Cochin appears to indicate increasing trends with respect to all pollutants. Delhi and Nagpur showed increased trends with respect to gaseous pollutants.
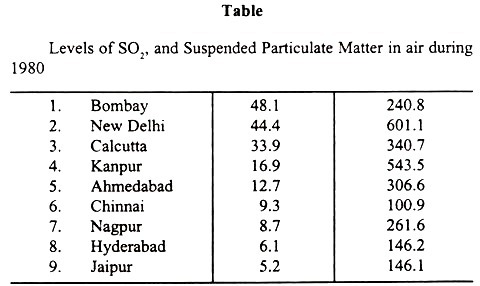
According to NEERI’s survey, Chembur- Bombay area of Bombay is having highest SO, pollution, while New Delhi is having highest air pollution of suspended particulate matter (SPM). In another survey, Calcutta is reported to have highest carbon monoxide pollution during peak traffic hours. The following Table indicates the levels of SO2 and suspended particulate matter (SPM) in air during 1980.
According to Prof. J.M. Dave, Dean of the School of Environmental Science at JNU and his team of researchers, 400 tonnes of pollutants are emitted every day in Delhi by nearly 500,000 vehicles according to 34 per cent of the smoke and dust emitted into the city.
According to a report prepared by the Institute of Petroleum, Dehradun, if vehicle pollution remains unchecked, the emission by vehicles in Bombay would reach the level of 1,07,000 tonnes of Carbon monoxide and 37,000 tonnes of hydrocarbons in early nineties and by 2000 AD, it has been estimated that 1,85,000 tonnes of Carbon monoxide and 6,93,000 tonnes of hydrocarbons will be released in Bombay city’s atmosphere.
Almost all north Indian cities undergo thermal inversion that makes the air nearest the ground colder than the air higher up. This makes the noxious gases which are driven up by the heating of the earth in the day time, descend once again at night.
What descends however, have been not the emissions of those night time hours alone, but of the entire day. In almost all cities, vehicular traffic pollution accounts for 50 per cent of the pollution and in some cities, it is even more. The problem of vehicular pollution in India is becoming worse day by day.
Adding to the problem is the pollution created by smoking almost in all places. Formerly, this was confined to developed countries alone. Now, this plague has caught hold of most of the Third World countries.
In India, smoking cigarettes and bidis has reached an uncontrolled level, causing health hazard, not only to the smokers, but also to no-smokers who become silent smokers due to the pollution of the atmosphere. At least 80 per cent of deaths due to lung cancer is the result of smoking. Nearly 75 per cent of smokers suffer from chronic bronchitis and emphysema and 25 per cent from Ischaemic heart disease.
More than 3,900 cities, towns and urban growth centres with more than one lakh population have begun experiencing the stress of air pollution. This valuable information would provide a tool for urban planners to locate residential, commercial and industrial zones suitably. It is essential that similar programmes should be organised on a continuing basis in all the other centres to aoid episodal events such as in Bhopal.
Bhopal Gas Tragedy (1984):
On December 2, 1984, Bhopal experienced the worst tragedy caused by massive leakage of Methyl-iso-cyanate (MIC) from the Union Carbide pesticide plant. MIC is a toxic gas used in the manufacture of pesticides. It reacts quickly with water and causes the lungs to swell and eyes to develop cataract.
This industrial accident which is related to air pollution resulted in many deaths. Estimates vary from 5,000 to 10,000 deaths. Nearly 2,00,000 people of Bhopal were affected and of which some 50,000 had been seriously affected and many had gone bline.
Apart from these massive casualties, children born to women who were pregnant, had either died or had deformities. It has been estimated that every 3 children born, only one survived and out of 1350 new born babies, 16 were physically deformed and 60 premature births.
Deformities include children suffering from cognitial hearts, holes in arms and impaired eyesight. High levels of thiocyanates were detected in water in Bhopal and continued exposure caused malfunctioning of organs, particularly thyroid glands which in turn affected pregnancy.
The vegetation in an area of 3.5 sq.km around the Union Carbide factory was severely affected. Cultivated plants and fruits were much affected than wild plants; and plants submerged in water were less affected.
Consumption of fruits like Mango, Papaya and tamarind was avoided by the people, as these plants were much affected by the pollution. The immediate cause of death for many people was due to formation of fluid in their lungs due to pollution of the air due to MIC leakage.
Essay # 7. Control of Air Pollution:
Air quality control is reduction of pollutants in the air by means of adopting efficient and modern technology. These control measures should be incorporated through appropriate legal methods, prescribing pollution control measures at the unit level in the case of ‘stationary’ sources and at the individual vehicle level in the case of’ mobile’ sources.
Minimum standards of air-quality should be prescribed. Effective monitoring of air should be undertaken periodically to ensure air quality. Enforcement of air-pollution control measures should be done irrespective of the sector in which the limit is functioning.
Generally in our country, the standards and implementation of law prescribed are enforced strictly in private sector units, whereas the government would be lukewarm in the case of public sector units. Glaring differences can be seen between the emission of heavily polluted smoke from public sector transport vehicles, maintained under public sector, as against private sector.
There are various procedures available to control air pollution, depending on the technology used. The first step in this is to contain the pollution to maximum extent possible by adopting appropriate engineering technology, so that the release of toxic substances from the exhausts and chimneys is considerably reduced.
The second process is to ‘replace’ or substitute old technology with new technologies by which there will be reduction of pollution. The third process is to have the released pollutants ineffective by means of dilution or self-cleansing, so that the concentration effects of pollution would be reduced.
Thus, the procedures of pollution control is a three-pronged drive, at the technological level, unit level and at the environmental level. All these procedures have to be effectively coordinated by means of legal policies and implementation of the laws relating to the pollution control. Let us discuss air-pollution control procedure indicated above, in a detailed manner with references to ‘mobile’ units and then ‘stationary units’.
a. Pollution Control in Automobiles:
In our country, the types of automobiles are: two-wheelers, three- wheelers, powered vehicles with two-stroke and four-stroke petrol engines and diesel engines trucks and buses, besides diesel engine units of the railways. In recent years, there had been spectacular increase in the number of these vehicles and also the extent of pollution caused by these in almost all cities, towns and semi-urban areas.
The extent of pollution from an automobile unit depends on the engine design, the type of fuel used, the extent of fuel consumption and also the operational efficiency of the engine.
Considerable amount of research work is going on in the modification of engine designs to make them more efficient to minimise emission of pollutants during combustion of gasoline. Similarly, fuel substitutes for gasoline would reduce concentration of pollutants.
It is found that methane, natural gas, reformed gasoline, blends of light hydrocarbons etc., could reduce noxious pollution in the exhaust gases. Some fuel additives, such as barium salts have been reported to give excellent results in reducing pollutants. Modification of existing fuels or introducing substitute fuels could be done after elaborate research and field trials about the toxicological contents of the exhaust fumes.
It is estimated that in India, an average emission of hydrocarbons from a passenger car is around 20 Kg per year and many methods are tried to control the evaporative emissions. In Japan, “Stratified Charge Engines” are developed.
These engines are provided with an additional chamber for combustion, where a fuel-rich mixture is introduced and ignited with spark. This sets a combustion at a relatively low temperature and as a consequence, the formation of NOx is greatly minimised.
Then the burning mixture is allowed to enter the large main chamber, where it gets mixed with lean-fuel mixture, i.e., air- fuel mixture with higher percentage of air and lesser percentage of fuel. This ensures complete combustion of CO and hydrocarbons without stopping the engine and at the same time NOx build up is limited due to lower temperature maintained in the chamber.
b. Pollution Control in Stationary Units:
In establishing a factory or an industrial unit, due assessment should be done about the probable extent of pollutants that would be discharged. If it is not conducive for the health of the air and environment, starting of factories or industrial plants should be totally prohibited in that region.
This decision has to be arrived at after carefully examining the meteorological conditions, humidity of the atmosphere and the type of environment.
The industrial units should be equipped with all modern devices to control gaseous pollutants and also particulate emissions.
In the control of gaseous pollutants, the following methods and equipment should be ensured in the industrial units:
(i) Combustion:
Good combustion should be ensured by having well equipped combustion chamber with adequate supply of oxygen to eliminate dark smoke with half burnt and un-burnt dust and ashes.
(ii) Absorption:
In this process, the gaseous effluents are passed through absorbers or scrubbers containing the suitable liquid to act as absorbent to remove or modify the pollutants present in the gas stream. The equipment used for this purpose may be of different kind ,, viz., Spray Towers, Plate Towers, Packed Towers, Liquid Jet Scrubber Towers etc.
The various absorbing liquids used are: water, alkaline water, sulphites of Calcium, Sodium or Barium etc., for absorbing SO2. For absorbing H2S, soda ash, ammonia liquor, sodium alamine, tripotassium phosphate etc., should be used. For NOx, water or NHO3, could be used.
(iii) Absorption:
In this, the gaseous effluents are passed through suitable porous solid absorbents in containers. Through interface between the effluent and the containers with chemical materials, the pollutant would get absorbed. Iron oxide for instance is used for absorbing H2S. Limestone pellets of NaF are used to absorb hydrogen fluoride.
Silica gel, activated alumina and synthetic zeolite could absorb water vapour from a mixture of water vapour and organic pollutants. Pulverized limestone or dolomite, alkalized alumina are used to absorb SO2. Petroleum fractions could be absorbed through bauxite.
Not only this, the emission of waste gases could be altogether prevented by re using them in the factory. If the waste gas contains higher concentrations of SO2, or NOx, the gases could be recovered and used for the manufacture of H2SO4 and HN03. Industrial chemistry would be much useful in controlling the pollution through appropriate absorbents.
In the case of particulate materials discharging and polluting, mechanical devices can be used. These devices depend upon the size, shape, electrical properties of the particulates.
These mechanical devices work on the principle of gravity setting in which the velocity of the gas is reduced, passing through a horizontal carrier and in the process, the particles settle down by gravitational force. Another method is to suddenly change the direction of the gas flow which causes the particles to separate out due to their greater momentum.
The most commonly used mechanical devices are: Buffer Chambers, Settling Chambers, and Cyclone Separators. In Cyclone Separators, the mechanical device consists of cylinder with a tangential inlet for gas entry and a vortex by which the suspended particles are collected and periodically removed.
There are wet cyclone collectors as well, for the removal of dissolved particulate matter; and in some plants, these collectors would be arranged in a series where 2 or 3 collectors would work, which facilitate removing particles of different size. By this method, nearly 90 percent of particulate matter could be removed.
Besides above mentioned mechanical devices there are other methods called (a) Filtration system (b) Electrostatic Precipitators and (c) Wet Scrubbers.
In the Filtration system, the particles are trapped in cloth-bag filters or in fabric filter media made from cotton, wool, nylon, asbestos, silicon coated glass cloth etc., depending on the nature and temperature of the particulates.
Electrostatic precipitators contain two electrodes which are insulated and electrically charged with differing potential. When the dust fumes are passed through this, the aerosol particles get precipitated on the electrode with lower potential.
This method will be very effective in the case of particles that could be electrically charged and this method is the best for carbons. Electrostatic precipitators can be used singly or in series or an additional equipment, besides cyclone collectors, so that when the gas reaches the chimney stacks, it will be almost pollution free, due to successive treatments. Electrostatic method will cost less, if the volume of gas to be handled happens to be very large.
Wet Scrubbers are also very effective devices, particularly when the temperature of the gases to be treated is as high as 300 degrees centigrade or more; when the gases happen to be combustible and cooling is desired and addition of water is not objectionable. Scrubbers are screens of water sprayed to remove large particles. Wet scrubbers are classified on the basis of the methods used in collecting particles.
These are Liquid Carriage type and Particle Conditioning type. In the former method, the gas is allowed to strike a liquid surface within the collector, and the liquids carrying the trapped gas particles are discharged in an outside collector, from where they are disposed off.
In the latter method, the dust particles in the gas stream are brought into intimate contact with water and the size of the panicles would be enlarged due to water-particulate agglomerates. These can be more easily removed. Besides, there are many other varieties of scrubbers called Gravity Spray Scrubber, Wet Centrifugal Scrubbers, Impinger Scrubbers etc.
Thus, there are various methods of reducing pollution and keeping the air clean. In all methods and programmes of air pollution control, care should be taken to see that they do not aggravate water pollution or solid waste pollution problems.
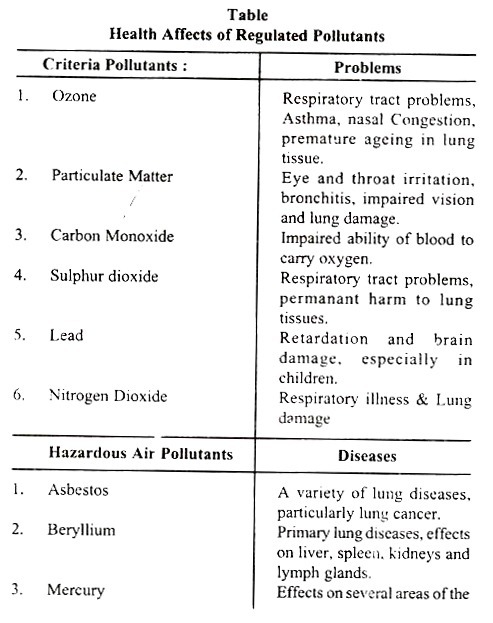
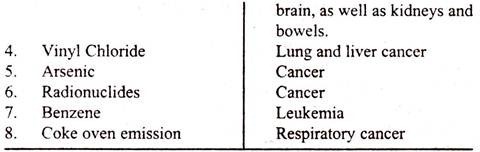
In USA, the Clean Air Act of 1970 authorised the Environmental Protection Agency (EPA) to prescribe and promote National Ambient Air Quality Standards. The Clean Air Acts regulated five pollutants called “Criteria Pollutants”, and in the year 1978, the list was increased by one more, i.e., Lead to make it six.
Besides six “Criteria Pollutants”, the Act regulated eight as “hazardous” air pollutants. According to EPA, the list of “Criteria Pollutants” and “hazardous” Pollutants and health effects are given in the table.
The EPA has established National Ambient Air Quality Standards for each of the six criteria pollutants. Consequent on the strict communities of air quality regulations and also the obedience of communities which do not violate the quality standards, there has been considerable decrease in pollution and the quality of ambient air has significantly increased during the last 20 years in USA. As per EPA’s report, there were considerable reduction in carbon monoxide emissions and nitrogen oxides.
But the most significant achievement has been a sharp decrease in air borne lead from a national emission of 23,000 tons annually in 1985 to 9,500 tons in 1985, a 59 per cent decrease achieved largely by continuing reductions in the lead content of gasoline. However, in the case of other pollutants, most of the urban areas are yet to reach the ambient air quality standards.
This is more so in the case of ozone pollution. According to EPA, 96 cities, countries, and other areas of urban settlement failed to meet federal ozone standards (1988) and more than 150 million Americans live in geographical areas that exceed the minimum safe levels of ozone exposure. Japan, UK and other countries also have enacted appropriate Air Pollution Legislations to ensure Ambient Air Quality.
In India also, the constitution provides for protection of environment through the 42nd Amendment in 1976. There are umpteen Acts to control pollution. It is not the number of legal provisions that are important, but the ability of the government to enforce the legislation effectively to realise the objectives of the Acts and also the character of citizens and their civic sense.
Essay # 8. How to Reduce Air Pollution?
To reduce the pollution load entering the environment several measures are taken:
a. Natural Cleaning of the Atmosphere:
Small particles in the air move around in the air like gas molecules. They collide with other particles and grow by coagulation. They ultimately fall down as large particles. Small particles also may fall within a raindrop and the raindrop also may collide and collect particles as it falls.
These processes are called rainout and washout. Gases also may be washed out by precipitation (absorption) from the atmosphere or they may be adsorbed (deposited) on solid particles and removed by gravity.
b. Source Correction:
The easiest solution to pollution problem is to stop producing the pollutant or to maintain an atmosphere in which pollutants have no negative impact on human activity. For example, lead emissions from automobiles are eliminated by burning non-leaded fuels. Similarly, nitrogen oxide emission can be significantly reduced by redesigning engines.
c. Control of Particulate Pollutants:
Designers of particle emission control equipment must deal with solid and liquid particles ranging from smaller than 1 pm to larger than 100 pm in diameter. The smaller particles are the most difficult to collect.
The important devices which are used to control particulate matters are:
i) Gravity settling chamber
ii) Centrifugal collectors (cyclone collectors and dynamic precipitator)
iii) Wet scrubbers (spray towers and venturi scrubbers)
iv) Electrostatic precipitators
v) Fabric filters.
d. Control of Gaseous Pollutants:
Generally there are four ways to reduce emission of undesirable gases:
i) Reduce or eliminate the production of the undesirable gases.
ii) Induce the gases to react after production in chemical processes to produce different, less objectionable emissions.
iii) Selectively remove the undesirable product from a gas stream by absorption (transfer of gas molecules into a liquid).
iv) Selectively remove the undesirable gas by adsorption (deposition of gas molecules on the solid surface).
The process by which the gas is recovered from the adsorbing solid or the absorbing liquid is called regenerative because the solid or liquid is used repeatedly in the same process.
e. Typical Recovery Processes:
As SO2 has effects on plants and human health and as H2S has foul odour even at very low concentrations, their removal or their reduce effects in industrial gas streams, is done by the following processes:
i) Selective removal of SO2 at high concentration in smelter gases.
ii) Selective removal of SO2 at low concentration from gas streams.
iii) Selective removal of H2S at high concentration from sour natural gas.
f. Nitrogen Oxide Emission Control:
Control of nitrogen oxide from combustion processes can be done by reducing the reacting temperature. In combustion processes, lower flame or combustion chamber temperature can be achieved by burning the fuel slowly and using multistage combustion. Recirculation of exhaust to dilute the fuel-air mixture in the combustion chamber has a similar effect.
g. Ambient Air Quality Control by Dilution:
Air pollution control processes have excessive cost for maintenance and operation, and high capital and interest charges. In air pollution it can be said that a substance is not a pollutant unless it causes an effect.
Thus, in the vicinity of the source, dilution is frequently used to achieve acceptable air quality. Dilution using tall stacks is a more economical way than installation of removal systems (cost effective) to attain an air quality standard.